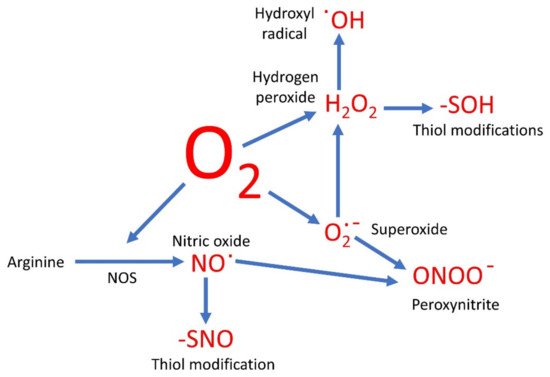
Oxygen-based compounds are an instrumental part of the group of small, relatively reactive molecules which control cellular activities. Traditionally such molecules have been referred to as the reactive oxygen species (ROS) and include hydrogen peroxide (H2O2), superoxide (O2∙−), and hydroxyl radicals (∙OH). However, several other reactive signaling molecules also contain oxygen, although referred to as reactive nitrogen species (RNS). These include nitric oxide (NO) and peroxynitrite (ONOO−), and therefore could be grouped together with the ROS as oxygen-based compounds.
1. Introduction
Historically, ROS were studied in biological systems as they are produced during a pathogen challenge, and it was suggested that their reactivity was harnessed to kill the invading organism [
2]. Studies concentrated on the production of ROS in phagocytic cells in animals, especially neutrophils, and the enzyme NADPH oxidase was characterized [
3]. This was aided by the realization that patients with Chronic Granulomatous Disease (CDG) had an impaired ROS generation and reduced pathogen tolerance. Interestingly, CGD can be inherited in both a X-linked and autosomal fashion, enabling the different NADPH oxidase components to be discovered [
4].
In 1987, it was reported that endothelial-derived relaxing factor (EDRF) was in fact the gas NO [
5]. Although this was not the first work on this gas in a biological setting, for example [
6], the 1987 paper did focus researchers’ efforts, and it was soon realized that other reactive compounds could partake in similar activities. NADPH oxidase homologues were reported in a range of cells suggesting a role in cell signaling reviewed in [
7]. Superoxide anions were to an extent ruled out as they were charged and deemed not able to pass through membranes, although once protonated this is not the case. Focus fell on H
2O
2, and ever since there has been an explosion of papers on this topic reviewed [
8,
9]. However, the efforts did not stop there, and now there are papers showing that a range of small molecules can partake in cell signaling events in an array of organisms. These molecules include others which contain oxygen, such as ONOO
− [
10] and carbon monoxide (CO) [
11], but also others which do not contain oxygen, including reactive sulfur species (RSS), such as hydrogen sulfide (H
2S) [
12], and hydrogen gas (H
2) [
13].
None of these molecules act in cell signaling events in isolation, and the interplay between them has been quite extensively reviewed [
14,
15,
16]. There is a complex interplay between them which creates downstream signals. As will be discussed below, many of these molecules are in competition with each other, potentially reacting with the same amino acid groups, such as thiols. It can, therefore, be seen that oxygen-based small molecules play a key part in the regulation of cellular function in a wide range of organisms.
2. Signaling by ROS
2.1. Superoxide and Its Role
Superoxide anions (O
2∙−) will be produced by the one electron reduction of molecular oxygen. The added electron leads to the molecule being both charged and a free radical, and therefore it is relatively reactive [
33]. Dismutation is likely in biological systems [
19] and is catalyzed by superoxide dismutases (SOD) [
34], producing H
2O
2. However, superoxide can be measured, and early work with neutrophils assayed O
2∙− by the reduction of cytochrome
c in the presence and absence of SOD, with a similar technique still being employed [
35].
One of the main sources of O
2∙− in cells is the family of NADPH oxidases [
20,
36,
37]. The oxidase from neutrophils was found to use NADPH as a cofactor, and on its oxidation, the electrons are sequentially passed to flavin, heme, and then oxygen. The enzyme had several subunits, including two in the membrane (gp91-
phox and p22-
phox) and several in the cytoplasm, which translocate to form a holistic enzyme. Among the cytoplasmic subunits is a G protein, although phosphorylation seems to also be important as part of the control mechanisms.
2.2. Hydrogen Peroxide as a Signal
The sequential oxidation of molecule oxygen produces O2∙−, then H2O2, and finally the hydroxyl radical (∙OH) before the four election reduction results in water. Therefore, once the O2∙− anion is formed, a cascade of further products is likely. As discussed below, there are side reactions likely here too. For example, hypochlorous acid can be produced in the presence of the enzyme myeloperoxidase [46].
H2O2 has been the focus of ROS signaling [8,51,52]. One of the ways in which H2O2 is known to alter cell function is by the oxidation of thiol groups in proteins [52], and such modifications can be analyzed by proteomic techniques [53,54]. The -SH group is converted to the sulfenic acid group, -SOH. This is in many ways akin to phosphorylation, and like phosphorylation, the formation of the -SOH group is likely to force a conformational change on the proteins and thus alter its activity. This is not necessarily activation. In tyrosine phosphatase, the interaction with H2O2 leads to the formation of a sulfenyl-amide intermediate and inhibition of the enzyme [55]. This means in the cell that the levels of tyrosine phosphorylation are likely to increase, with the concomitant effects that leads to.
2.3. Hydroxyl Radicals Can Be Signals Too
Hydroxyl radicals (
∙OH) are often produced in the presence of other ROS via the Fenton reaction [
74] or the Haber–Weiss reaction [
75]. Transition metals are therefore important for the formation of
∙OH in cells. The formation of
∙OH has been discussed by others [
76,
77].
∙OH are extremely reactive and therefore not thought to be very functional as a signal. However, there are a range of papers which show that this molecule does have a role in controlling cell function [
78].
∙OH has been shown to be involved in ion movements in plant roots [
79,
80] and the control of kinase pathways [
81]. This radical is also involved in mitochondrial oxidative stress [
82] and cytoplasmic oxidative stress [
83], and to participate in the modification of proteins and lipids and polysaccharides [
84,
85,
86].
There is no doubt that
∙OH can be detected in cells [
87], and their modulation has been suggested as beneficial [
88,
89], not just because they do damage but because they have a positive influence.
3. Signaling by RNS
3.1. Nitric Oxide and Working with Other Oxygen-Based Molecules
NO has been found to be involved in the mediation of a wide range of biological functions, from controlled blood flow in humans [100], to controlling stomatal apertures in plants [101]. In animals, the main source is NOS. In humans, there are three isoforms of this enzyme: eNOS, iNOS, and nNOS [102]. However, the existence of such an enzyme in plants has been hotly contested and it is unlikely to exist, at least in the form that would be easily recognizable [96]. It is more likely that in plants the main source of NO is the enzyme nitrate reductase (NR) [103], although as mentioned above there are other sources of NO in biological systems.
A universal mechanism of NO signaling is the modification of protein thiol groups, in what has been dubbed S-nitrosylation [106]. However, this terminology is technically incorrect, and this modification should preferably be called S-nitrosation [107]. Either way, this is the formation of the -SNO group, and like the formation of -SOH by H2O2, this formation of -SNO causes a conformational change on the protein and therefore a modulation of its activity or function. As this is a reversable reaction it is again akin to phosphorylation. However, the thiols are also able to be oxidized, as discussed, so there is likely to be competition for the thiol between the oxidation by ROS and nitrosation by NO. Furthermore, the same thiols may be under attack by H2S, in S-sulfhydration [108,109], as well as being able to be glutathionylated [110]. Which thiol modification actually results depends on the environment of the thiol and the relative concentrations of the molecule trying to attack it. As many of these reactions are reversible, the whole system is likely to be very dynamic, allowing different modifications happening with time and in different locations.
Proteins can also be nitrated on tyrosine. Therefore, NO can mediate the modification of polypeptides in more than one manner [111], and such changes are not mutually exclusive.
Last, NO can partake in some direct reactions with other important redox molecules. One of the most significant is the generation of S-nitrosoglutathione (GSNO). This not only removes glutathione from its important role as a redox mediator, especially in ROS metabolism, as discussed above [42], but it also creates a new signaling molecule. It has been suggested that GSNO is a buffer for NO, GSNO formation being reversed by S-nitrosoglutathione reductase (GSNOR) [112], but it may also be able to be moved around an organism in the vasculature [113], so allowing long-range NO signaling. NO can also react with H2S in the formation of nitrosothiol, which can act as a signal as well [114].
3.2. Peroxynitrite, as a Signal
The effects of peroxynitrite accumulation are mediated by tyrosine nitration of proteins [
116]. Peroxynitrite can also react with amino acids, such as cysteine, methionine, and tryptophan [
117]. As well as amino acids, RNA nitration is also possible [
118]. Through nitration reactions, as well as oxidation effects, peroxynitrite can alter the phosphorylation levels in cells, by affecting both kinases and phosphatase activities [
119], which would have significant consequences for signal transduction pathways. A profound effect of peroxynitrite can be seen in its control of the intrinsic apoptosis pathway, mediated by MAPK and Akt signaling [
120], which would lead to cell death.
Therefore, peroxynitrite may have effects on signaling, but may be a significant mediator of NO signaling pathways, especially if ROS are accumulating spatially and temporally together with NO.
4. The Signaling of Carbon Monoxide
Many of the effects of CO are mediated through the action of heme oxygenase [121,122]. This enzyme degrades heme to produce biliverdin, ferrous ions, and CO.
In a similar manner to ROS and RNS, CO is inherently toxic [123]. It can inhibit the activity of Complex IV of the mitochondrial ETC, for example. Even so, as it can inherently interact with metal containing proteins, it is known to modulate the activities of several enzymes, and this can lead to changes to the accumulation of ROS and NO. It can also alter cGMP levels, an instrumental intracellular signaling molecule. Furthermore, CO effects can be mediated by MAPK pathways and by changes in the activity of ion channels [124]. One of the mechanisms of action of H2 is thought to be mediated by heme oxygenase [125], which would then impinge on CO signaling.
5. Conclusions
Evolution would have started in the absence of oxygen, but as the atmospheric oxygen concentration increased, this relatively reactive di-radical, and products which could be generated, had to be tolerated. Many of the compounds to which cells became exposed would have been toxic, including ROS, RNS, and H
2S. Instead of simply managing the presence of these molecules, cells adapted to adopt these compounds as signal molecules, and many are now instrumental in the control of cellular activity [
127]. Interestingly, they are often involved in stress responses, being produced by cells deliberately. Furthermore, such generation of these molecules is often spatial and temporally the same. This can lead to competitions and interactions between them, making the downstream effects often difficult to unravel.
Evolution has therefore resulted in the use of a range of cell signaling molecules which are both instrumental to cellular control and contain oxygen (Figure 1). These may be reduced states of molecular oxygen, or have oxygen covalently bonded to nitrogen (NO) or carbon (CO). Each of these has potentially different roles in the cell, but they rarely work in isolation.
Figure 1. A simplified overview of oxygen-based molecules in signaling. Oxygen and oxygen-based signaling molecules are shown in red.
The accumulation of ROS and RNS has been implicated in normal and dysfunctional cellular function. Overaccumulation of ROS leads to what is referred to as oxidative stress [
8,
9].
Some of the products of what appears to be scavenging have a useful signaling role. The production of H
2O
2, for example, may be important, allowing signaling that O
2∙− may not be able to mediate. The reaction of glutathione with NO can lead to
S-nitrosoglutathione (GSNO) [
112], which may be able to move around an organism giving long-distance signaling which NO would not be able to partake in owing to its reactivity [
113].
Cells, however, need to control the accumulation of these reactive molecules but still allow their concentrations to transiently rise to a level which allows them to signal to the next component in the cell’s signal transduction pathway. To do this, compartmentalization is almost certainly the key [
44].
In conclusion, there are a range of oxygen-based small, and often relatively reactive, molecules which are instrumental to signaling in cells. This applies across the kingdoms of organisms, from prokaryotes [
139], through plants and animals to humans. These reactive molecules have a complex interplay which can lead to a range of responses. Metabolic enzymes, such as GAPDH [
57], as well as gene expression [
51] may be controlled by these molecules. The production of ROS, RSS, and RNS was not only tolerated by organisms during the early stages of evolution, but they have since been adopted as instrumental signaling components [
127]. A better understanding of how the balance and compartmentalization of these molecules is achieved in cells, along with the pathologies and diseases in which they are involved, will allow such metabolism to be better controlled, with the concomitant benefits that will bring. There is still much research to carry out to measure the spatial and temporal accumulation of these molecules, and it is becoming more apparent that they should not be studied in isolation, but a holistic view of oxygen-based signaling molecules should, be taken.
This entry is adapted from the peer-reviewed paper 10.3390/oxygen1010002