1. Introduction
Bordetella pertussis produces several virulence factors. These could be classified into two main groups as adhesins and toxins. Several of the adhesins are components of the current acellular pertussis vaccines (ACV) in various compositions. These are filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae (FIM). Most of the toxins are proteins such as pertussis toxin (PT), adenylate cyclase toxin, and dermonecrotic toxin. Non-protein toxins are tracheal cytotoxin, which is a fragment of the peptidoglycan, and lipo-oligosaccharide (endotoxin). Detoxified PT is the major component of all current ACVs.
PT is a major virulence factor of
B. pertussis [
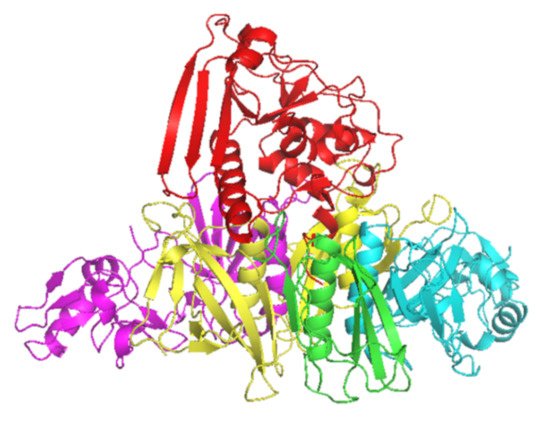
1] and is a combination of five subunits (S1–5) (
Figure 1). S1 has ADP-ribosyltransferase activity using NAD as ADP-ribosyl donor and signal transduction, G proteins as ADP-ribosyl acceptors [
2]. PT is secreted through the
B. pertussis cell membrane to its surroundings, and as a consequence of the PT actions in the intracellular processes, there will be a regulatory imbalance such as the uncontrolled formation of cAMP, which will lead to metabolic changes and paralysis in the target cells. The S2–5 binds to various (but mostly unidentified) glycoconjugate molecules on the surfaces of eukaryotic cells, and is involved in the translocation of the toxic S1 across the cell membrane [
3]. Historically, before the identification of actions of just one protein, PT had several names that reflect its different toxic properties: islet-activating protein, histamine-sensitizing factor, and lymphocytosis-promoting factor. Additionally, PT is also a mitogen and an adjuvant [
4].
Figure 1. Crystal structure of PT subunits 1 (red), 2 (purple), 3 (cyan), 4 (yellow) and 5 (green). Generated by PyMOL (version 2.3.1, Schrödinger, LLC).
In 1974, Yuji Sato and Hiroko Sato reported the isolation and characterization of protective antigens of
B. pertussis tested in mice [
5]. This led to the development and use of ACVs, which mainly contained formalin detoxified PT and FHA. ACVs have been used in Japan since 1981. One of the main advantages of ACVs, as compared to whole-cell vaccines (WCV), is the removal of endotoxin, which is the main cause of fever and other side effects related to earlier vaccines. Locht et al., first described, cloned, and sequenced the PT gene, which was later confirmed by the group of Rino Rappuoli [
4,
6]. A new genetically inactivated recombinant PT vaccine was developed, in which the enzymatic activity was inactivated through precise genetic modifications. Later, the recombinant PT vaccine was combined with FHA and PRN and was shown to be immunogenic and safe in infants, and induced early and long-lasting protection [
7,
8,
9].
One problem in the understanding of pertussis pathophysiology is the lack of biomarkers, which indicate protection against the disease. Hemagglutinating antigens (PT and FHA) are important virulence factors [
5]. Historically, it is known that high levels of agglutinating antibodies are protective [
10]. Agglutinin response is related to FHA, lipo-oligosaccharide, PRN, and FIM2 and 3. Results from household studies in Sweden and Germany indicate that antibodies against PT and PRN correlate with protection [
10,
11,
12]. Most likely, the protection is multifactorial and is related to both humoral and cellular immunity both in local mucosal surfaces and in systemic immune systems [
13,
14,
15,
16].
There is a two-phase decay of antibodies against PT, both after disease and after immunization. In Denmark, Dalby et al., showed that the biphasic decay, consisting of a rapid decay followed by a slower long-lasting decay, was faster after the disease (median half-life 221 days) than after adult booster vaccination with hydrogen peroxide inactivated pertussis vaccine (median half-life 508 days). The decay of antibodies after the disease was not age-related [
17]. Hallander et al., (2005) studied the decay after the last dose of primary immunization in infants (2-, 4-, 6-month schedule) during the Swedish vaccine trials with chemically detoxified ACVs. The initial rapid decay occurred in 8–9 months, and in 65 months 50% of the children had undetectable antibodies against PT [
18].
Denmark is the only country where protection against pertussis is nationally provided with a hydrogen peroxide inactivated single pertussis antigen vaccine, which was started in 1997. In 2003, a five-year booster was introduced. After 2005, the situation of pertussis seemed to be rather stable (population-level incidence < 10/100.000) until outbreaks occurred in 2012, 2016, and 2019 [
19,
20]. The incidence of pertussis in infants has been much higher than in the neighboring Nordic countries, and one reason is speculated to be the lack of adolescent boosters. However, it should be kept in mind that direct comparisons between countries with different surveillance systems for pertussis incidence are challenging [
20,
21]. Temporary maternal immunizations were introduced in Denmark in November 2019 after a nationwide epidemic started in July (
Statens Serum Newsletter, No 42/43—2019). However, in 2019 Denmark also changed to an ACV vaccine containing PT and FHA (
https://en.ssi.dk/news/news/2019/new-vaccine-formulation-in-the-childhood-vaccination-programme, accessed on 15 May 2021).
The problem with all pertussis vaccines has been the relatively rapid waning of immunity, especially against PT. It was also found that the immunogenicity of ACVs is higher in infants than in adolescents [
16,
18,
22]. Since the enzymatic activity of native PT is detoxified for use in ACVs, most often chemically with formaldehyde, glutaraldehyde, or hydrogen peroxide, it has been proposed that the repeating use of ACVs induces B cells preferentially recognizing PT epitopes, which are induced by the chemical treatment of the toxin rather than epitopes against the native PT [
23]. Thereafter, new, live attenuated bacterial vaccines were developed for intranasal use to mimic natural infection [
24]. During active development, new vaccines with several different preventive strategies will be needed, and these should be tested in different age groups. The change to new vaccines is not going to be easy, since pertussis vaccines are one of the key components in the basic primary immunization of all children, globally.
A great number of studies are focused on the quantity of anti-PT IgG antibodies created after vaccination or infection. It is commonly accepted that a high quantity of anti-PT antibodies should give enough protection against the disease [
11,
25]. However, this approach does not describe the functionality of the antibodies. In order to provide more definitive evidence for vaccination- and infection-induced PT-specific antibodies’ ability to prevent the diverse biological activities of this protein, different methods have been developed in the field. These methods include, e.g., the neutralization of leukocytosis-promoting activity, antibody-mediated opsonophagocytosis [
26], and importantly the measurement of the neutralization capacity of anti-PT antibodies, which can be evaluated by the antibodies’ ability to prevent the clustering of Chinese hamster ovary cells [
27,
28,
29]. Other general aspects, such as the affinity of antibodies and their specific binding locations to PT, may act as contributive factors in the antibodies’ potency to neutralize and clear out the toxin [
30,
31]. In addition, it is crucial to understand the true nature of the machinery to produce these antibodies such as B cell populations, including memory B cells (Bmem), behind the antibody production and how they change during the lifespan [
32,
33]. Our aim in this review is to focus on functional antibodies to PT and their impact on improved understanding of anti-PT antibodies after vaccination and infection.
2. The Role of Neutralizing Antibodies to Pertussis Toxin (PTNAs)
Neutralization capacity of vaccine-induced and naturally boosted antibodies is commonly evaluated for virological or toxin-mediated bacterial studies to conclude good protection against the target pathogen [
34,
35,
36,
37,
38]. In regard to pertussis studies, this approach has been used to study the quantity of toxin-neutralizing antibodies. However, it has not been successfully demonstrated that neutralizing antibodies would act as a correlate of protection against the disease in humans [
39,
40]. A commonly used approach to measure neutralizing capacity of anti-PT antibodies is based on the clustering effect and form changes of Chinese hamster ovary (CHO) epithelial cells. This method was originally used to study the activity of other AB
5 toxins such as cholera toxin [
41]. During the 1980s, the first CHO cell studies regarding PT were published, and they proved to be comparable to results from other toxins with similar CHO cell-clustering effects [
27,
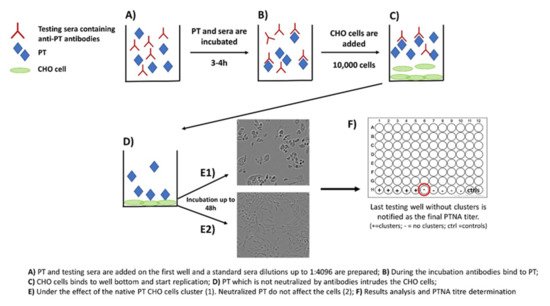
28]. The method includes the following steps: (1) PT and serially diluted sera are first mixed together on a microwell plate; (2) the plate is incubated for 2–4 h; (3) a known number of CHO cells are added on the wells; and (4) the plate is monitored for up to 48 h for the formation of CHO cell clustering (
Figure 2). The final neutralizing titer is notified in the well without any clusters [
28,
29].
Figure 2. Principle of the CHO cell-based assay for neutralizing antibodies to pertussis toxin.
The CHO cell assay has been widely used to study PT activity within pertussis vaccines to show that PT is properly detoxified [
7,
23,
42,
43]. Furthermore, it has also been used to show the protective properties of monoclonal antibodies (Mabs) targeted to different epitopes of PT subunits [
5,
44]. These epitope studies highly increased our knowledge of structure-based protection against PT and are described in more detail within the section of “PT-specific epitopes after infection and vaccination“.
However, none of the epitope-specific neutralization studies describe how the results were related to actual protection in humans acquired from vaccination or infection. Granström et al., and Trollfors et al., performed the first studies concerning this aspect. They showed a clear increase of the neutralizing titers (≥4-fold) after clinical infection, which remained high up to two years in patients that could be followed, despite a rapid initial decrease [
45,
46]. Soon after this, Granström et al., focused on serological correlates for protection against pertussis with pre-disease samples. According to their results, both antibody titers and neutralizing capacity indicated that anti-PT antibodies are crucial for protection [
40]. Podda et al., focused on childhood vaccination and showed how PTNA titers increased from 1.1 (pre-vaccination) to 269.1 (after three doses). Interestingly, the titer increased highly after the second dose, whereas after the first dose there was almost no increase in the titer, an observation that also was shared in other studies [
8,
47]. These neutralizing antibodies were still detectable after five years [
48]. Both chemically detoxified and genetically modified PT vaccines have demonstrated high post-vaccination neutralizing antibodies after one month, whereas WCVs induce a considerably weaker response, which was attributed across all studies to be closely related with the overall capability to induce high anti-PT IgG titers [
23,
49,
50,
51,
52,
53,
54,
55].
Another approach to study PTNAs was a PBMC-based method in which neutralization was monitored through the mitogenic activity of PT. These results were compared to the CHO cell assay. The investigators showed increased titers either post-vaccination or after infection compared to pre-vaccination sera. They also plotted anti-PT ELISA titers against CHO/PBMC assay titers, but the correlation was poor for both assays (r
2 = 0.37/0.02, respectively). Therefore, the authors speculated that the ELISA, CHO, and PBMC assays measure distinct fractions of anti-PT antibodies [
56]. In contrast, the majority of CHO-cell-based assays have shown a clear correlation (r > 0.80) between the concentration of anti-PT IgG antibodies and CHO cell neutralizing titers [
46,
47,
48,
49,
55,
57,
58,
59], concluding that basic ELISA measurements could be used to demonstrate the neutralization capacity of antibodies [
48,
57,
59]. Furthermore, most of the neutralization and protection is based on anti-PT IgG antibodies, whereas IgA and IgM play a minor role [
59]. Another novel study regarding PTNAs in infected subjects concluded a similar correlation between the quantity of anti-PT IgG antibodies and neutralizing titers (r = 0.68). A good correlation was found among those previously vaccinated (r = 0.71) subjects, but within the unvaccinated group with pertussis, a poor correlation (r = 0.26) was noticed. This also indicates good memory of the vaccine-based response [
29]. Furthermore, a Japanese seroprevalence study among the heathy population classified the ratio between neutralizing titers and total IgG antibodies as low (<0.6), mediocre (0.6–<1.8), and high responses (≥1.8). The group of <0.6 ratio (cases with lower neutralization than expected based on anti-PT IgG) was the most common (over 60%, N = 106). Although the distribution of subjects into these three categories was similar between among groups, the correlations between CHO cell titers and anti-PT IgGs, decreased with age [
60].
In the light of different studies performed, PTNAs do correlate with the total amount of anti-PT IgG antibodies and are induced considerably both after infection and vaccination, but they also indicate that high antibody concentration does not always guarantee good neutralization capacity [
29,
47,
55,
57]. Although these studies increase our understanding of PT antibody-based protection, further modifications for future testing might be needed to better show the actual fraction of neutralizing antibodies after vaccination or natural infection. Since the CHO cell assay is laborious, a high throughput assay should be developed in the future. Moreover, how to define a good neutralization capacity is another question to be considered. The CHO cell assay only measures the ability of antibodies to block the intrusion of PT into these cells. Thereafter, other abilities of the antibodies not covered in this review, e.g., to prevent leucocytosis, opsonophagocytosis and bactericidal capability are other critical lines of interest [
26,
61,
62].