Normal wound healing cascade is highly dynamic and has four distinct overlapping phases which involves several cellular and molecular interactions. It is known to be one of the most complicated processes in human body. The wound healing mechanism can be interrupted due to the involvement of several diseases that eventually develop to chronic wounds such as in diabetic foot ulcers. Infection is a common problem in chronic wound cause by microbes residing on the superficial layer of the skin. It is frequently resulting in impaired wound healing and patient morbidity and mortality. Antibiotic therapy and wound dressings are the main treatments to treat infected chronic wounds. However, the presence of polymicrobial infections, formation of bacterial biofilms and antibiotics resistance are the major challenges faced by healthcare providers to kill or eliminate the microbes from the wounds. Considering all the possible factors, more studies are needed to elucidate the role of microbes as well as the selection of suitable empirical antibiotics in reducing the infections and provide optimal healing in chronic wounds.
- normal flora
- microbes
- wound healing
- chronic wound
- wound infection
1. Introduction
Skin wound healing is a highly complex and dynamic mechanism involving various regulatory cells and molecules integrating to complete the wound re-epithelialization cascade [1]. Once the cutaneous layer is broken, the embedded cellular and molecular substances within the skin layers will synchronize at the designated phases to initiate the healing mechanism. Even though cutaneous wound healing is a systematic process, the phases are overlapping; therefore, it is known as one of the most complicated biological processes in human body [2]. A chronic wound can be described as a stalled wound or wound that cannot heal in the expected time frame which is less than 3 months [3].
A chronic wound is caused by a local factor (infection), systemic factor (diabetes), or both [4]. Generally, chronic wound is a healthcare and socioeconomic burden. Approximately 2% of the population in developed countries has the potential to develop chronic wounds, especially leg ulcers, once in a lifetime [5]. Chronic wounds affected around 5.7 million people in the USA alone, at the cost of $20 billion for treatment and management yearly. The incidence is predicted to rise significantly in the elderly due to diabetes [6]. Besides skin lesion and diabetes, there are various underlying diseases such as sickle cell anemia, calciphylaxis, systemic lupus erythematosus (SLE), skin disease, or impaired physiological states that include paralysis, malnourishment (lack of nutrients), aging, and poor mobility that can affect the sequence of healing events, resulting in non-healing or chronic wounds [7].
In diabetes, for instance, several complications can lead to chronic wounds. However, diabetic foot ulcer (DFU) is considered more alarming than any other complications as it has become the primary cause of morbidity and increased hospital care for diabetic patients [8]. In addition, poor vascular flow and life-threatening infections are the major causative factors in diabetic chronic wounds impairing the wound healing process [9]. According to Armstrong and his team (2017), the cost of treatment for diabetic foot care has exceeded the cost for common cancers, as the diabetic wound is responsible for more admissions than any other diabetic complications. The researchers further stated that diabetic patients who developed foot ulcerations are at two times higher risk of death within 5 years upon diagnosis than patients who are not diagnosed with foot ulcers [10].
Based on the 2015 prevalence data, the International Diabetes Federation (IDF) reported that approximately 463 million adults are living with diabetes in 2019 [11], and diabetic foot with lower extremity complications affects about 40 to 60 million people globally (International Diabetes Federation-Complications 2020). In addition, the World Health Organization (WHO) has estimated that diabetes will be the seventh foremost death cause in 2030 [12]. Recently, it has been reported that every 30 s, one leg is being amputated due to DFU in some part of the world [13].
2. The role of normal flora as protective agents toward skin
Normal flora play important function to maintain homeostasis and maturation of the skin. There are around 1000 types of normal flora inhabiting the human skin. Normal flora are referred as bacterial normal flora since bacteria typically populate the human skin without causing any harm to healthy individuals [14]. Most of the bacterial normal flora live on the surface layer of the epidermis and the upper parts of the hair follicles [15] while some reside beneath the hair follicles. These bacteria are the reservoir for re-colonization after the removal of the surface bacteria [16]. The most abundant types of bacteria that take up the huge space of the skin are Staphylococcus epidermidis [17] and Staphylococcus aureus [18], Staphylococcus haemolyticus, Staphylococcus hominis [19], and Micrococci species [20].
The relationship between bacterial normal flora and host are either mutualistic or commensalism, and once established settlement, the microbial communities remain constant and stable on the skin over time [21] Some factors could cause interruption to normal flora-host relationship such as age, gender, medications (antibiotics), disease, different geographical areas and lifestyles [22],[23]. A slight alterations caused by any of the abovementioned factors could become favouritism for some bacteria to colonize the skin more than the others. An example is lifestyle, particularly hygiene and use of cosmetics, soaps and other body products influence the dynamic of normal flora by removing them from the skin and encourage other microbes to grow [24] [25]. Normal flora protect the skin barrier from pathogens invasion. For an example, S. epidermidis induce the activation of CD8 T cells with IL-17A or IFN-γ to inhibit the entry of pathogens into the skin [26]. Besides that, S. epidermidis also release substances known as phenol soluble modulins (PSMs) and bacteriocin which is harmful and prevent the multiplication of microbes on the skin surface [27].
3. The role of microbes in acute wound healing
Similar to other living organisms, microorganisms need essential requirements such as suitable temperature, pH, and nutrients for survival and growth [28]. The normal pH of healthy skin for both genders is between 4 to 5, which is acidic [29]. The acidic condition is one of the way to protect the skin from exogenous microorganisms [30]. The acidic pH of the skin is a desirable condition for S. epidermidis to preserve and attach to the skin, whereby this condition is unfavorable or could inhibit the growth of some skin pathogens such as S. aureus and P. acne. Most skin pathogens prefer alkaline environments [31]. In acute wound healing, when the wound becomes acidic, the amount of lactic acid and oxygen will reduce the pH of the skin. Acidic condition of the wound is needed for macrophages response, multiplication of fibroblasts, angiogenesis, collagen synthesis, and DNA formation to facilitate wound closure. In contrast, a wound that develops infection appears to increase in an alkalinity environment [32][33].
Recent studies have found that S. epidermidis stimulate CD8 T cells to cytotoxic T cells (CTLs) through RNA gene sequencing. The studies further revealed that immune regulation and genes associated with tissue repair were up-regulated. They concluded that both bacteria and T cells not only prevent foreign particles invasion but also hastening skin wound healing [34][35]. Moreover, a similar finding has been proven in skin biopsy samples whereby induced wound injury exposed to S. epidermidis drastically improved re-epithelialization and tissue granulation [34]. Figure 1 of this review provides an overview of factors that contribute to wound healing.
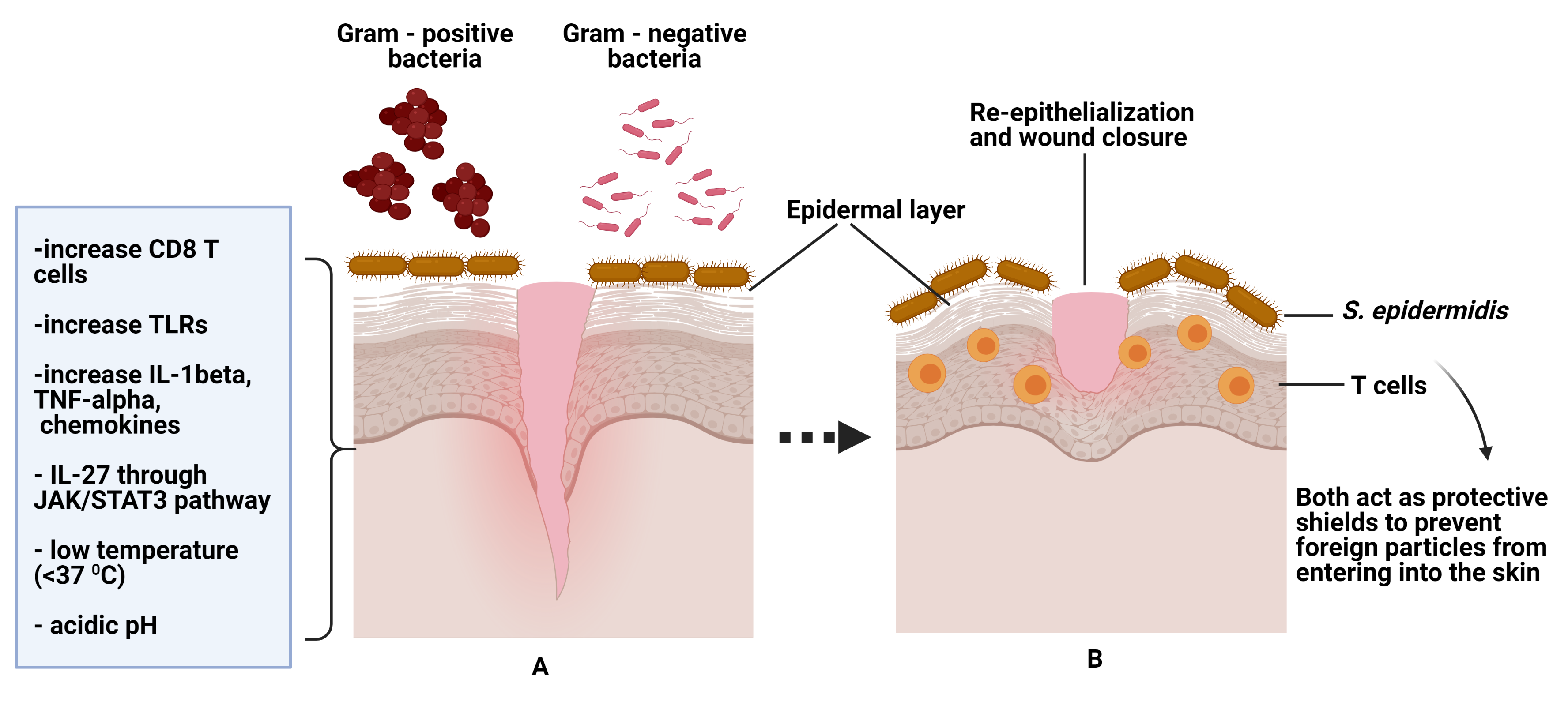 Figure 1. (A) Figure A shows normal flora/skin microbiome (S. epidermidis) on normal wound healing. The skin is protected from gram-positive /negative bacteria by S. epidermidis. The left corner shows factors that contribute to wound healing and further preventing colonization by skin pathogens. (B) Figure B shows both S. epidermidis and T cells help in wound closure and act as protective shields against pathogen invasions
Figure 1. (A) Figure A shows normal flora/skin microbiome (S. epidermidis) on normal wound healing. The skin is protected from gram-positive /negative bacteria by S. epidermidis. The left corner shows factors that contribute to wound healing and further preventing colonization by skin pathogens. (B) Figure B shows both S. epidermidis and T cells help in wound closure and act as protective shields against pathogen invasions
4.The role of microbes in chronic wound healing
There are few types of microorganisms that are accountable for infections in chronic wounds which include Staphylococcus aureus, Staphylococcus epidermidis (both gram-positive bacteria) while Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Enterobacter species, and Morganella species (gram-negative bacteria). Among the microbes, S. aureus mostly resistant to antibiotics and contains high virulence, contributing to the pathogenicity of the host [36].
The bacteria that already occupied the wounded sheet generally produce biofilms acting as barrier to allow them to grow, multiply, and protect from immune cells or become resistant to the antibiotics. The biofilm architecture comprises a fraction of bacteria implanted in the extracellular polysaccharide matrix or extracellular polymeric substances (EPS). Additionally, this biofilm is toxic to the other skin cells, explaining the delay in wound healing [37][38][39]. The EPS is water-based, containing a matrix with some protein substances that help channel nutrients, movement, and communication between the bacterial communities in a biofilm. In brief, EPS is the main component of most bacterial biofilms that support colonization or recolonization, by adhering to the wounded surface area [40][41].
Bacterial biofilm formation is one of the indicators for its presence in a chronic wound with more than 50% are detectable through microscopic observation compared to only 6% in an acute wound. The bacteria species and the relative number varied from one wound to another [42][43]. Some anaerobic bacteria can survive and multiply deeper in a biofilm even though the oxygen level is depleted. Therefore, it is vital to investigate the type of bacterial strains and their relationship within the wound since it is insufficient to kill the biofilm cells by looking into the bacterial colonies alone [43].
Neutrophils utilize neutrophil extracellular traps (NETs) to kill infectious microbes or biofilms by releasing chromatins and granular protein contents. It is also known as NETosis.[44]. In addition, S. aureus is able to induce extracellular trap formation by releasing leucocidin from the biofilm to escape the antimicrobial activity of NETs. This encourages the multiplication of bacterial colonies to disperse to a new place for new biofilm formation; thus, helps the bacteria to sustain and survive in chronic wound for a longer time [45]. Figure 2 of this review provides an overview of favourable conditions for pathogens' survival and factors that cause cell death in chronic wound.
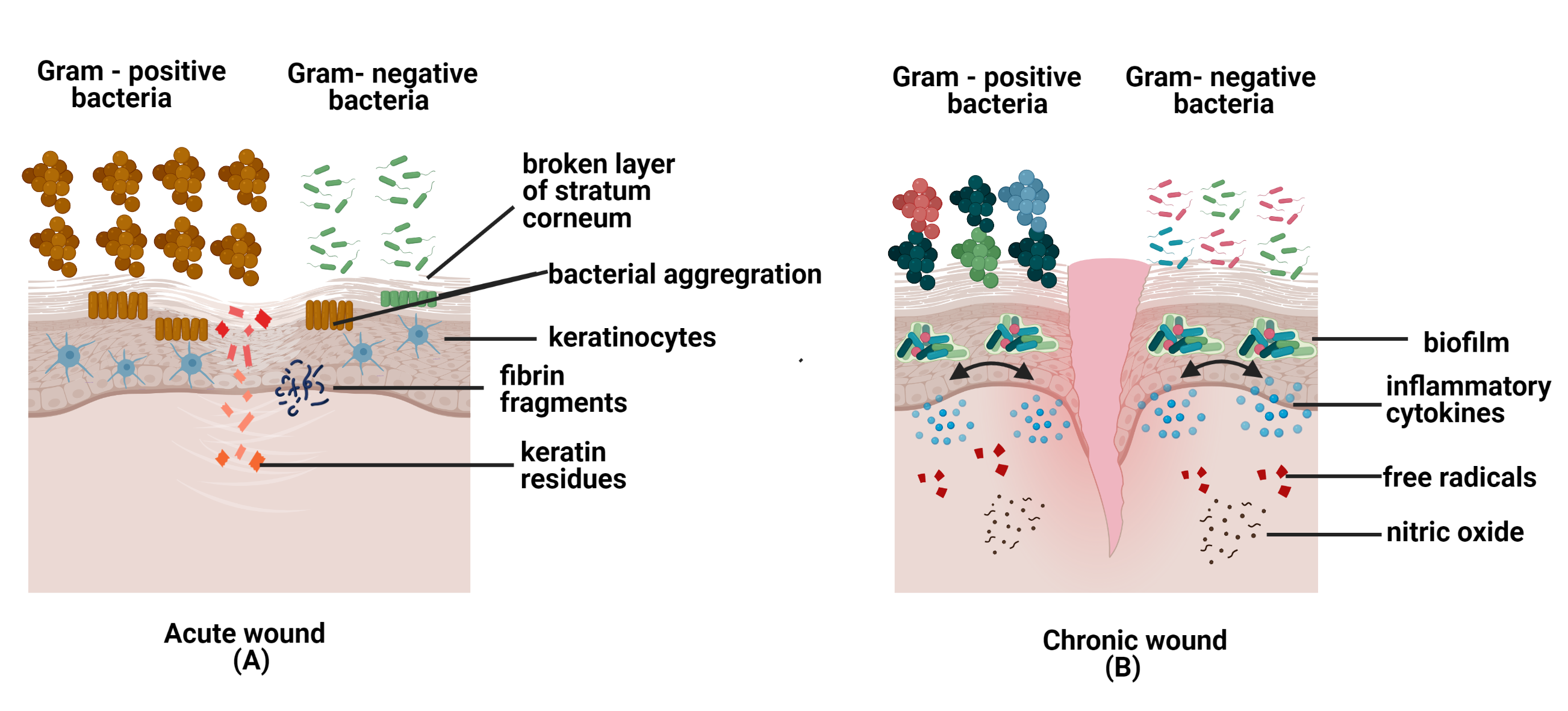
Figure 2. The figures above show microbes in acute and chronic wounds. (A) In acute wounds, fibrin fragments and keratin residues are favorable conditions for pathogens invasion into the skin. Hence, bacteria aggregate to prevent immune cells from destroying the bacterial community; (B) In chronic wounds, the biofilm formation secretes inflammatory cytokines, free radicals, and nitric oxide, which are toxic to cells and cell apoptosis.
5. Treatments for chronic wounds and diabetic foot ulcers
Antibiotic treatments can be administered to the patients either by parenteral, oral, or topical [46]. Since some antibiotics can reduce a type of pathogen while increasing the growth of others, an empirical antibiotic choice should be carefully selected based on the clinical examinations, the severity of the infection, antimicrobial sensitivity pattern, and the aetiological agent. A broad spectrum of parenteral antibiotics is administered for severe infections, while narrow-spectrum oral antibiotics are administered for mild infections [47].
Besides drugs, there are different types of wound dressings applied for wound treatments such as passive dressings, interactive dressings, advanced dressings, bioactive dressings, and antimicrobial dressings. The goal of a good dressing is to retain moisture for wound closure, prevent infections, reduce pain or irritation, and scar formation [48].
References
- George Han; Roger Ceilley; Chronic Wound Healing: A Review of Current Management and Treatments. Advances in Therapy 2017, 34, 599-610, 10.1007/s12325-017-0478-y.
- Melanie Rodrigues; Nina Kosaric; Clark A. Bonham; Geoffrey C. Gurtner; Wound Healing: A Cellular Perspective. Physiological Reviews 2019, 99, 665-706, 10.1152/physrev.00067.2017.
- Anna, L.-R.; Fabrellas, N.; Saez Rubio, G.; Wilson, K; Time of chronic wound healing , as part of a prevalence and incidence study. Enfermeria Global 2017, 16, 445-453, org/10.6018/eglobal.16.2.251311 .
- Eoghan Mulholland; Nicholas Dunne; Helen O. McCarthy; MicroRNA as Therapeutic Targets for Chronic Wound Healing. Molecular Therapy - Nucleic Acids 2017, 8, 46-55, 10.1016/j.omtn.2017.06.003.
- Myriam Le Goff-Pronost; Bénédicte Mourgeon; Jean-Pierre Blanchère; Luc Téot; Hervé Benateau; Anne Dompmartin; Real-World Clinical Evaluation And Costs Of Telemedicine For Chronic Wound Management. International Journal of Technology Assessment in Health Care 2018, 34, 567-575, 10.1017/S0266462318000685.
- Brown, M.S.; Ashley, B.; Koh, A; Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Frontiers in Bioengineering and Biotechnology 2018, 6, 47, 10.3389/fbioe.2018.00047.
- Samuel R. Nussbaum; Marissa J. Carter; Caroline E. Fife; Joan DaVanzo; Randall Haught; Marcia Nusgart; Donna Cartwright; An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value in Health 2018, 21, 27-32, 10.1016/j.jval.2017.07.007.
- Sandhu, D.K.S.; Singh, D.K.; Banga, D.R.K.; Sandhu, D.K.S.; Samria, D.J; Role of topical phenytoin (Diphenylhydantoin) dressing in diabetic ulcers: A comparative study with conventional dressing. International Journal of Orthopaedics Sciences 2018, 4, 239-242, 10.22271/ortho.2018.v4.i1d.35.
- Perez-Favila, A.; Martinez-Fierro, M.L.; Rodriguez-Lazalde, J.G.; Cid-Baez, M.A.; Zamudio-Osuna, M.D.J.; Mar-tinez-Blanco, M.; Mollinedo-Montaño, F.E.; Rodriguez-Sanchez, I.P.; Castañeda-Miranda, R.; Garza-Veloz, I; et al. Current therapeutic strategies in diabetic foot ulcers. Medicina 2019, 55, 714, 10.3390/medicina55110714.
- David G. Armstrong; Andrew J.M. Boulton; Sicco A. Bus; Diabetic Foot Ulcers and Their Recurrence. New England Journal of Medicine 2017, 376, 2367-2375, 10.1056/nejmra1615439.
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R; Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice 2020, 162, 108086, 10.1016/j.diabres.2020.108086.
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D; Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomedicine and Pharmacotherapy 2019, 112, 108615, 10.1016/j.biopha.2019.108615.
- Daba Abdissa; Tesfaye Adugna; Urge Gerema; Diriba Dereje; Prevalence of Diabetic Foot Ulcer and Associated Factors among Adult Diabetic Patients on Follow-Up Clinic at Jimma Medical Center, Southwest Ethiopia, 2019: An Institutional-Based Cross-Sectional Study. Journal of Diabetes Research 2020, 2020, 1-6, 10.1155/2020/4106383.
- Chen, P.; He, G.; Qian, J.; Zhan, Y.; Xiao, R; Potential role of the skin microbiota in Inflammatory skin diseases. Journal of Cosmetic Dermatology 2021, 20, 400-409, 10.1111/jocd.13538.
- Mia Maguire; Greg Maguire; The role of microbiota, and probiotics and prebiotics in skin health. Archives of Dermatological Research 2017, 309, 411-421, 10.1007/s00403-017-1750-3.
- Bujung Hong; Andreas Winkel; Philipp Ertl; Sascha Nico Stumpp; Kerstin Schwabe; Meike Stiesch; Joachim K. Krauss; Bacterial colonisation of suture material after routine neurosurgical procedures: relevance for wound infection. Acta Neurochirurgica 2017, 160, 497-503, 10.1007/s00701-017-3404-9.
- Teruaki Nakatsuji; Tiffany H. Chen; Anna M. Butcher; Lynnie L. Trzoss; Sang-Jip Nam; Karina T. Shirakawa; Wei Zhou; Julia Oh; Michael Otto; William Fenical; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Science Advances 2018, 4, eaao4502, 10.1126/sciadv.aao4502.
- Jasim, H.A; Study of Type of Bacteria That Found in Ear, Mouth, Nose of Second Stage Nursing Department Student. Indian Journal of Forensic Medicine & Toxicology 2020, 14, 2789-2793, 10.37506/ijfmt.v14i4.12012.
- Vikas Gautam; Nandini Sethuraman; Ramanpreet Kaur; Suchet Sachdev; Neelam Marwaha; Pallab Ray; Changing epidemiology of coagulase-negative staphylococci in normal flora of skin.. Indian Journal of Medical Microbiology 2017, 35, 277-278, 10.4103/ijmm.IJMM.
- Ashwag Shami; Samiah Al-Mijalli; Pisut Pongchaikul; Ahmed Al-Barrag; Samah Abdurahim; The prevalence of the culturable human skin aerobic bacteria in Riyadh, Saudi Arabia. BMC Microbiology 2019, 19, 189, 10.1186/s12866-019-1569-5.
- Yiyin Erin Chen; Michael A. Fischbach; Yasmine Belkaid; Skin microbiota–host interactions. Nature 2018, 553, 427-436, 10.1038/nature25177.
- Stegli, A.; Jachowicz, A.; Justyna, S.; Szulc, J.; Adamiak, J.; Otlewska, A.; Pielech-Przybylska, K.; Gutarowska, B; Factors Influencing Microbiological Biodiversity of Human Foot Skin. International Journal of Environmental Research and Public Health 2019, 16, 3503, 10.3390/ijerph16183503.
- Anthony M. Cundell; Microbial Ecology of the Human Skin. Microbial Ecology 2016, 76, 113-120, 10.1007/s00248-016-0789-6.
- Mathilde Fournière; Thomas Latire; Djouhar Souak; Marc Feuilloley; Gilles Bedoux; Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752, 10.3390/microorganisms8111752.
- Christopher Wallen-Russell; Sam Wallen-Russell; Meta Analysis of Skin Microbiome: New Link between Skin Microbiota Diversity and Skin Health with Proposal to Use This as a Future Mechanism to Determine Whether Cosmetic Products Damage the Skin. Cosmetics 2017, 4, 14, 10.3390/cosmetics4020014.
- Samantha R. Ellis; Mimi Nguyen; Alexandra R. Vaughn; Manisha Notay; Waqas A. Burney; Simran Sandhu; Raja K. Sivamani; The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550, 10.3390/microorganisms7110550.
- K. Szabó; L. Erdei; B. Sz. Bolla; G. Tax; T. Bíró; L. Kemény; Factors shaping the composition of the cutaneous microbiota. British Journal of Dermatology 2016, 176, 344-351, 10.1111/bjd.14967.
- Yasmine Belkaid; Julia A. Segre; Dialogue between skin microbiota and immunity. Science 2014, 346, 954-959, 10.1126/science.1260144.
- Eung Ho Choi; Gender, Age, and Ethnicity as Factors That Can Influence Skin pH. Metabolic Disorders and Nutrition Correlated with Skin 2018, 54, 48-53, 10.1159/000489517.
- Adawiyah Jamil; Choon S Lee; Skin pH and its relationship with transepidermal water loss and disease severity in children with atopic dermatitis: A cross-sectional study. Journal of Dermatology and Dermatologic Surgery 2020, 24, 84, 10.4103/jdds.jdds_33_20.
- Blaak, J.; Staib, P; The Relation of pH and Skin Cleansing. Issues and Challenges 2018, 54, 132-142, 10.1159/000489527.
- Bennison, L.R.; Miller, C.N.; Summers, R.J.; Minnis, A.M.B.; Sussman, G.; McGuiness, W.; The pH of wounds during healing and infection : A descriptive literature review. Journal of the Australian Wound Management Association 2017, 25, 63-69, 10.3316/informit.927380056251808.
- Carla R. Kruse; Mansher Singh; Stefan Targosinski; Indranil Sinha; Jens Ahm Sørensen; Elof Eriksson; Kristo Nuutila; The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair and Regeneration 2017, 25, 260-269, 10.1111/wrr.12526.
- Caroline Leonel; Isadora Fernandes Gilson Sena; Walison N. Silva; Pedro H. D. M. Prazeres; Gabriel Da Rocha Fernandes; Pamela Mancha Agresti; Mariana Martins Drumond; Akiva Mintz; Vasco A. C. Azevedo; Alexander Birbrair; et al. Staphylococcus epidermidis role in the skin microenvironment. Journal of Cellular and Molecular Medicine 2019, 23, 5949-5955, 10.1111/jcmm.14415.
- Apollo Stacy; Yasmine Belkaid; Microbial guardians of skin health. Science 2019, 363, 227-228, 10.1126/science.aat4326.
- Alavi, S.M.; Khosravi, A.D.; Abdullah, S.; Dashtebozorg, A.; Montazeri, E.A; Bacteriologic Study of Diabetic Foot Ulcer. Pakistan Journal Medical Science 2007, 23, 681-684, 10.1016/j.ijid.2008.05.519.
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M; Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, Causes, and Approaches to Care. Advances in Skin and Wound Care 2012, 25, 349-370, 10.1097/01.ASW.0000418541.31366.a3.
- Kashif Rahim; Shamim Saleha; Xudong Zhu; Liang Huo; Abdul Basit; Octavio Luiz Franco; Bacterial Contribution in Chronicity of Wounds. Microbial Ecology 2016, 73, 710-721, 10.1007/s00248-016-0867-9.
- Ning Xu Landén; Dongqing Li; Mona Ståhle; Transition from inflammation to proliferation: a critical step during wound healing. Cellular and Molecular Life Sciences 2016, 73, 3861-3885, 10.1007/s00018-016-2268-0.
- 125. Li, Y.; Wang, Y.; Zhou, L.; Liu, M.; Liang, G.; Yan, R.; Jiang, Y.; Hao, J.; Zhang, X.; Hu, X.; et al. V γ 4 T cells inhibit the Pro-healing Functions of Dendritic epidermal T cells to Delay skin Wound closure Through IL-17A. Frontiers in Immunology 2018, 9, 240, 10.3389/fimmu.2018.00240.
- Flemming, H.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Biofilms : An emergent form of bacterial life. Nature Reviews. Microbiology 2016, 14, 563-575, 10.1038/nrmicro.2016.94.
- M. Malone; T. Bjarnsholt; Andrew McBain; G.A. James; Paul Stoodley; D. Leaper; M. Tachi; G. Schultz; T. Swanson; R.D. Wolcott; et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. Journal of Wound Care 2017, 26, 20-25, 10.12968/jowc.2017.26.1.20.
- Amin Omar; J. Barry Wright; Gregory Schultz; Robert Burrell; Patricia Nadworny; Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9, 10.3390/microorganisms5010009.
- Maren Von Köckritz-Blickwede; Stefanie Blodkamp; Victor Nizet; Interaction of Bacterial Exotoxins with Neutrophil Extracellular Traps: Impact for the Infected Host. Frontiers in Microbiology 2016, 7, 402, 10.3389/fmicb.2016.00402.
- Mohini Bhattacharya; Evelien T. M. Berends; Rita Chan; Elizabeth Schwab; Sashwati Roy; Chandan K. Sen; Victor Torres; Daniel J. Wozniak; Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proceedings of the National Academy of Sciences 2018, 115, 7416-7421, 10.1073/pnas.1721949115.
- Huidi Tchero; Pauline Kangambega; Lazarre Noubou; Beatrice Becsangele; Sergiu Fluieraru; Luc Teot; Antibiotic therapy of diabetic foot infections: A systematic review of randomized controlled trials. Wound Repair and Regeneration 2018, 26, 381-391, 10.1111/wrr.12649.
- Ki Tae Kwon; David G. Armstrong; Microbiology and Antimicrobial Therapy for Diabetic Foot Infections. Infection & Chemotherapy 2018, 50, 11-20, 10.3947/ic.2018.50.1.11.
- Matthew S. Brown; Brandon Ashley; Ahyeon Koh; Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Frontiers in Bioengineering and Biotechnology 2018, 6, 47, 10.3389/fbioe.2018.00047.
