Nonalcoholic fatty liver disease (NAFLD) encompasses a broad spectrum of pathological hepatic conditions ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), which may predispose to liver cirrhosis and hepatocellular carcinoma (HCC). The pathogenesis of NAFLD is closely related to insulin resistance (IR), adiposity and physical inactivity as well as genetic and epigenetic factors corroborate to the development and progression of hepatic steatosis and liver injury. Emerging evidence has outlined the implication of gut microbiota and gut-derived endotoxins as actively contributors to NAFLD pathophysiology probably due to the tight anatomo-functional crosstalk between the gut and the liver. Obesity, nutrition and environmental factors might alter intestinal permeability producing a favorable micro-environment for bacterial overgrowth, mucosal inflammation and translocation of both invasive pathogens and harmful byproducts, which, in turn, influence hepatic fat composition and exacerbated pro-inflammatory and fibrotic processes.
1. Introduction
The global burden of nonalcoholic fatty liver disease (NAFLD) as the leading cause of chronic liver disorders represents a major concern for public health. It encompasses a wide spectrum of hepatic conditions ranging from simple steatosis, a benign manifestation characterized by lipid accumulation exceeding 5% of liver weight excluding other etiological causes, to a more severe form, such as nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis and hepatocellular carcinoma (HCC) [
1,
2]. NAFLD is broadly spread in Western countries, affecting between 20% and 40% of the adult population, possibly due to the epidemics of obesity and type 2 diabetes mellitus (T2DM) [
3].
The pathogenesis of NAFLD is closely intertwined with increased adiposity, insulin resistance (IR) and dyslipidemia [
4]. Dietary factors such as excessive caloric intake, fructose and physical inactivity represent other risk factors for this condition [
5]. Furthermore, the inter-individual variability in NAFLD phenotype may be at least in part attributed to genetics. Single nucleotide polymorphisms (SNPs) in proteins regulating hepatocellular lipid handling, including Patatin-like Phospholipase Domain-containing 3 (PNPLA3), Transmembrane 6 Superfamily Member 2 (TM6SF2) and Membrane Bound O-acyltransferase Domain-containing 7 (MBOAT7), have been associated to NAFLD predisposition and progression towards NASH and fibrosis [
6]. However, less than 10% of inherited variability is explained by these common variants. Many of the phenotypic differences may also result from gene-environment interactions, referred to as epigenetics, a hereditable but reversible phenomenon that affects gene expression without modifying DNA sequence, such as alterations of DNA nucleotides (i.e., methylation), modifications of histones and regulation of transcription by altering mRNA stability through small RNA molecules such as microRNAs (miRNAs) [
7,
8].
Therefore, as a complex disease, the pathophysiology of NAFLD is not completely elucidated and may be simultaneously influenced by multiple parallel hits including IR, oxidative stress, inflammation, epigenetic modifiers and many others. Among the plethora of risk factors, recent evidence has pointed out to the role of gut microbiota and its metabolites in the pathophysiology of alcoholic fatty liver disease (ALD) and NAFLD [
9,
10]. Indeed, qualitative and quantitative changes in gut microbiome composition (referred to as ‘dysbiosis’) and derangement in the gut–liver axis that favors viable gut-derived bacteria and endotoxins translocation into the bloodstream have emerged to be independently associated to the development of NAFLD and its progression to NASH and HCC. Thus, species-specific microbial communities might profile NAFLD stages [
11,
12,
13,
14,
15], possibly enabling the intestinal flora modulation a diagnostic strategy and an eventual therapeutic intervention in the personalized NAFLD management. Currently, liver biopsy remains the gold standard procedure for diagnosis of NASH and no medications have been approved for the treatment of NAFLD patients except for modifications in lifestyle, nutrition and physical exercise and weight loss [
16,
17].
2. Insight into the Gut Microbiota in NAFLD
The human gastrointestinal lumen is the physiological habitat for more than 100 trillion microorganisms, which is approximately ten-times the number of somatic cells in the human body, hosting a wide variety of microbial species (archaea, fungi, yeast, bacteria and viruses) [
16]. The gut microflora is a large reservoir of commensal microbes that live synergistically with the host and provide biological and metabolic functions benefiting the host. It includes more than 160 different bacterial species, including anaerobes and they carry more than three million unique genes [
17,
18]. Among them, bacteria predominate with the phyla of the Gram-positive Firmicutes and Gram-negative Bacteroidetes, mainly involved in the short-chain fatty acids (SCFAs), i.e., acetate, butyrate and propionate and hydrogen production, respectively. The other phyla are represented by Actinobacteria, Fusobacteria, Proteobacteria and Verrucomicrobia [
19,
20,
21]. The precise function of the intestinal flora remains largely uncharted. However, it processes complexed and indigestible polysaccharides to SCFAs, providing energy to the host and it also participates in vitamin (i.e., vitamin B and K), bile acid and amino acid synthesis, drug and toxin metabolism and intestinal barrier preservation. In particular, the term ‘dysbiosis’ indicates all imbalances between beneficial and pathogen bacteria or modifications in intestinal flora taxonomic composition and/or function [
22]. Perturbations in intestinal microbiota homeostasis has been already described not only in NAFLD, but also in ALD [
10], T2DM [
23], obesity [
24,
25] and many other diseases [
26,
27,
28,
29].
Along the gastrointestinal tract (GIT) from the mouth to colon, the bacterial concentration and composition is strikingly diverse (increasing from stomach to colon), showing even higher variability depending on the age, lifestyle, medications and diets. Indeed, a diet enriched in animal fat and sugars as well as the Western diet may predispose to bacterial overgrowth, immune system activation and mucosal inflammation both in preclinical [
30,
31] and clinical studies [
32,
33].
Several approaches have been developed to study the intestinal flora community diversity, exploiting quantitative real time polymerase chain reaction (qRT-PCR), sequencing of the 16S ribosomal RNA (rRNA) gene through next-generation DNA sequencing or partial 16S rRNA sequencing in the V6–V8 region through pyrosequencing, excepting for Enterobacteriaceae and Enterococcaceae families [
34]. These tools provide information about the abundance and the taxonomy of microbial species in mucosa-associated colonic tissue biopsies and in fecal samples. All these techniques are also coupled with the more expensive metagenomics or metatranscriptomics shotgun approaches [
35]. Nonetheless, to study the host-microbiome interactions, intestinal, systemic, uric, and fecal bacterial-products and metabolites, such as bile acids, SCFAs and endotoxins, can be assessed by using proteomic and metabolomic methods and may represent diagnostic noninvasive markers, reflecting the microbiota composition [
36].
3. Gut–Liver Axis: New Awareness in NAFLD Pathogenesis and Progression
The gut–liver axis has many implications in NAFLD onset as the major contributor of the intestinal dysbiosis, possibly due to the tight anatomo-functional crosstalk of the two organs. The liver is perpetually exposed to gut microbial end-products and nutrients via the portal vein (70% of blood supply) and, in turn, participates to bacterial composition through bile acids cycling released into the duodenum lumen with the enterohepatic circulation [
10]. Alongside, the gut microbiome composition is crucial to modulate innate and adaptive immune response both locally and systemically, facilitating host defense against pathogens.
The bowel wall plays an essential role as a selective barrier that regulates the bidirectional flux between the gut and the liver, since it is constituted by tight and adherent junctions (occludins, claudins and Zonula Occludens 1 (ZO-1)) and desmosomes, which hold together the epithelial cells. Furthermore, it exerts many immunological functions as it is constituted by multiple layers and specialized cells, such as Goblet, Paneth and plasma cells secreting mucus, antimicrobial peptides (i.e., defensins, lysozyme and c-lectin Reg3b/g) and Immunoglobulin A (IgA), respectively. Together they protect the host from invasive pathogens and avoid bacterial overgrowth and systemic translocation [
10]. The excessive erosion of the protective mucus layer as well as the reduction of antimicrobial mediators has been associated with translocation of pathogenic microorganisms in both preclinical and human studies [
70,
71].
Disturbance of the intestinal barrier integrity, a phenomenon known as leaky gut, along with shifting in metabolic function of gut microbiota, are frequently present in patients with NAFLD-related dysbiosis [
51,
72] and correlate with NAFLD severity. Indeed, a relative abundance in Bacteroides and Ruminococcus have been independently associated with NASH and fibrosis [
73]. As a consequence of enhanced gut permeability, much more bacteria and potentially harmful byproducts translocate into circulation and reach the liver thus contributing to the increase of circulating gut-derived toxins (endotoxemia) and the establishment of chronic low-grade inflammatory state that features metabolic disorders such as obesity and NAFLD [
74,
75]. Several endogenous molecules as ethanol, ammonia and acetaldehyde, whose circulating increased levels result from dysbiotic microbiota (i.e.,
Escherichia coli abundance), are able to stimulate hepatic Kupffer cells to produce pro-inflammatory cytokines with similar mechanisms occurring in ALD [
10,
57]. Likewise, LPS and peptidoglycans derived from Gram-negative and Gram-positive bacteria walls are the most representative pathogen-associated molecular patterns (PAMPs), which activate Toll-like receptors (TLRs) signaling. In particular, LPS-induced TLR-4 cascade in hepatocytes, Kupffer cells and hepatic stellate cells (HSCs) leading to elevated systemic levels of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL6) via nuclear receptor kappa B (NF-kB) thus promoting IR, inflammation and fibrosis [
9,
76]. Otherwise, circulating free fatty acids (FFAs), whose levels are commonly higher in NAFLD, may independently stimulate TLR4 and TLR2 inflammatory pathways [
77,
78]. Furthermore, peptidoglycans and damage-associated molecular patterns (DAMPs) contribute to liver damage through the crosstalk between TLRs (e.g., TLR2 and TLR5) and inflammasome via intracellular nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), which increase IL1 and IL8 production in hepatocytes, Kupffer cells and HSCs [
60].
Moreover, alteration of gut microflora communities contributes to liver pathology and disruption of intestinal barrier integrity. For instance, dysbiosis may affect lipid metabolism and trafficking in both liver and adipose tissue by upregulating lipogenic enzymes or lipoprotein lipase (LPL) thus participating to obesity and steatosis development. Interestingly, several intestinal bacteria species dampen the production of the Fating-Induced Adipocyte Factor (FIAF), whose downregulation is associated with increased adiposity and hepatic de novo lipogenesis [
79]. Enrichment in Cytophaga–Flavobacter–Bacteroides phyla influences the development of fatty liver and hepatic inflammation favoring IL7 release from T-helper cells (Th17) [
80]. Dietary choline is further metabolized by enteric bacteria in trimethylamine and then it is converted in the hepatotoxic trimethylamine N-oxide (TMAO) end-product. Indeed, choline shortage or increased TMAO production have been associated with higher levels of Gram-negative Gammaproteobacteria and Erysipelotrichi and with steatosis since its levels are crucial to favor very-low density lipoprotein (VLDL) assembly and secretion.
Microbial SCFAs may affect the intestinal barrier integrity and mucosal immune tolerance raising levels of intestinal SCFAs-producing species strengthen barrier integrity supporting tight junctions and mucins production and operating as energy source for intestinal mucosal cells [
81,
82]. For example, the reduction of produce butyric acid, produced by
Faecalibacterium prausnitzii, weakens the few connections between intestinal epithelial cells, by decreasing the expression of the tight junction proteins and mucins. The restoration of the physiological abundance of microorganisms-producing butyrate, in turn, may ameliorate the gut high permeability and systemic inflammation [
83].
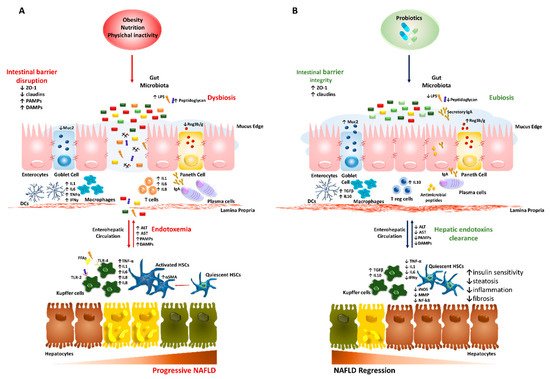
The molecular features of the gut–liver axis in NAFLD/NASH are schematically represented in Figure 1A.
Figure 1. Role of the gut–liver axis in progressive nonalcoholic fatty liver disease (NAFLD) and probiotic-related beneficial mechanisms. We summarized the main abnormalities involved in dysbiotic NAFLD and the impact of probiotic treatment on the gut–liver axis. (A) Obesity, diet and physical inactivity favor hepatic steatosis, disruption of the intestinal barrier and alter microflora taxonomic composition (dysbiosis). This condition may promote gut hypermeability through loosening tight junction proteins (ZO-1 and claudins), thinning mucus layer (Muc2), reducing antimicrobial peptides and IgA secretion. The leaky gut stimulates a pro-inflammatory local milieu, recruiting macrophages, dendritic cells (DCs), T CD4+ and T CD8+ as well as promoting phenotype switch of B cells into plasma cells. This pathologic microenvironment facilitates the systemic translocation of pathogenic bacteria and gut-derived pathogen-associated molecular patterns (PAMPs)/damage-associated molecular patterns (DAMPs; such as lipopolysaccharides (LPS) and peptidoglycans). Upon arrival to the liver via portal vein, PAMPs/DAMPs and high levels of free fatty acids (FFAs) activate inflammatory response in hepatocytes, Kupffer cells and hepatic stellate cells (HSCs) through Toll-like receptors (TLRs) cascade, which enhances the release of cytokines and chemokines (such as TNFα, IL1, IL6, IL8 and IFN-γ) and worsens liver damage. (B) Probiotics may restore intestinal barrier integrity, positively acting on ZO-1 expression, mucus thickness and commensal bacteria proportion (eubiosis). Moreover, they participate to the shutdown of bowel inflammation, enrolling T regulatory cells, DCs and macrophages to secrete anti-inflammatory cytokines (TGF-β and IL10). In the liver, the reduction of endotoxemia halts hepatic damage, as shown by lower aminotransferases (ALT and AST) and contributes to the recovery of the hepatic functions, affecting the lipid composition of fatty-laden hepatocytes, favoring endotoxins clearance, as well as negatively impacting on inflammatory and fibrogenic processes (i.e., lower iNOS, MMP and NF-kB).
4. Probiotics: Cunning Double-Crossers Against Their Household
Current interventions for the management of NAFLD focused on dietary and lifestyle modifications, although the discouraging results due to the poor compliance of patients. In addition, hypolipidemic drugs, anti-TNFα, antioxidants and diabetes medications have been proposed for NAFLD/NASH, even though no pharmacological therapies or surgical procedures have been approved for the treatment of NAFLD. In the last decade, intensive efforts have been directed to develop new strategies targeting the gut–liver axis as it appears as an attractive converging point for the prevention of NAFLD onset and/or progression. Several approaches to modulate dysbiosis include 1) untargeted methods (diet, probiotics, prebiotics, antibiotics and fecal microbiota transplant (FMT)) or 2) microbiota-targeted therapy (MTT) which selectively target microbial and host metabolites [
109].
The mechanisms by which unbalancing in gut microbiota participate to liver pathology remains still uncertain; however promising results on modulation of intestinal flora have been reported in several preclinical and human studies. Increasing efforts have been addressed to exploit the ability of probiotics to reverse gut dysbiosis and only recently they have been proposed as treatment of NAFLD.
Probiotics are defined as a “live microorganism that—when administered in adequate amounts—confer a health benefit on the host” by the World Health Organization/Food and Agriculture Organization (WHO/FAO). The criteria for the selection of probiotic strains are represented by the safety (i.e., absence of genes responsible for antibiotic resistance), functionality (i.e., resistance of lower pH in the stomach) and technological usability (i.e., high survival rate in finished products) [
110]. Among them, commercialized
Streptococcus/Lactobacillus/Bifidobacteria promote anti-inflammatory environment and help intestinal epithelium growth and survival as well as they may counteract the pathogenic bacteria by modulating immune system and host defense [
111].
The present chapter would deeply highlight the most recent findings on health benefits gained with probiotic administration in the experimental models of NAFLD and in the clinical practice.
The probiotics benefits on the gut–liver axis in NAFLD/NASH are summarized in Figure 1B.
This entry is adapted from the peer-reviewed paper 10.3390/nu11112642