Migraines are a common disease with limited treatment options and some dietary factors are recognized to trigger headaches. Although migraine pathogenesis is not completely known, aberrant DNA methylation has been reported to be associated with its occurrence. Folate, an essential micronutrient involved in one-carbon metabolism and DNA methylation, was shown to have beneficial effects on migraines.
- epigenetic diet
- DNA methylation
- folate
- migraine
- valproic acid
1. Introduction
2. Epigenetic Regulation of Gene Expression
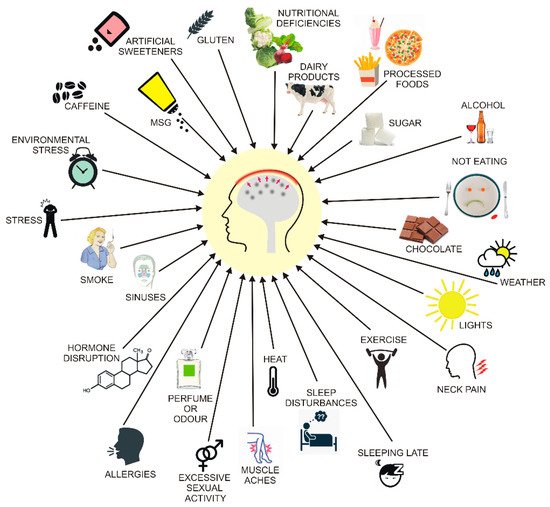
3. Migraine and Diet
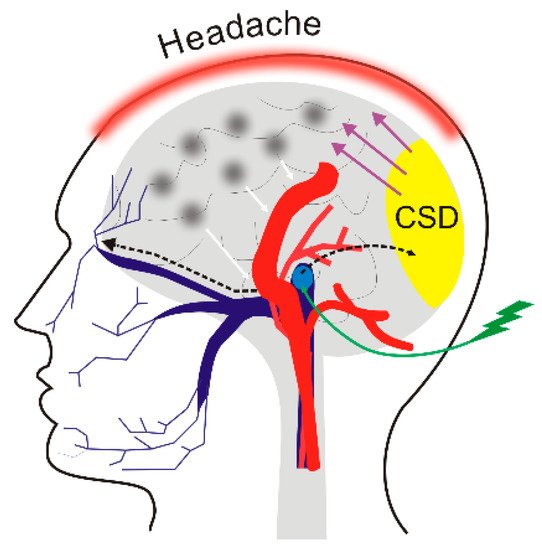
4. DNA Methylation in Migraine
|
Full Name |
Reference |
|
|---|---|---|
|
SH2D5 |
SH2 domain containing 5 |
[72] |
|
COMT * |
catechol-O-methyltransferase |
[73] |
|
ZNF234 |
zinc finger protein 234 |
[73] |
|
SOCS1 |
suppressor of cytokine signaling 1 |
[73] |
|
SLC2A9, SLC38A4, SLC6A5 |
solute carrier family 2,38A,6A member 9,4,5 |
[76] |
|
DGKG |
diacylglycerol kinase gamma |
[76] |
|
KIF26A |
kinesin family member 26A |
[76] |
|
DOCK6 |
dedicator of cytokinesis 6 |
[76] |
|
CFD |
complement factor D |
[76] |
|
RAMP1 * |
receptor activity modifying protein 1 |
[77] |
|
CGRP * |
calcitonin gene related peptide |
[80] |
|
CRCP *1) |
CGRP receptor component |
[80] |
|
CALCRL *1) |
calcitonin receptor like receptor |
[80] |
|
ESR1 *1) |
estrogen receptor 1 |
[80] |
|
NOS3 *1) |
nitric oxide synthase 3 |
[80] |
* denotes genes reported previously to associate with migraine, 1) DNA methylation studied in rats.
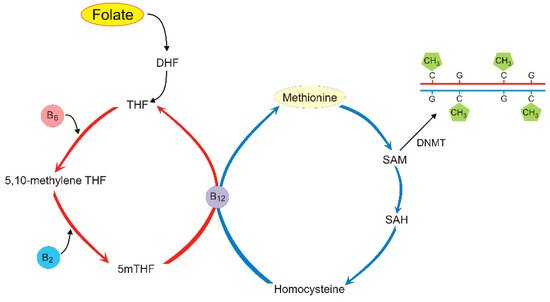
5. Folate and Its Role in DNA Methylation and Migraine Pathogenesis
This entry is adapted from the peer-reviewed paper 10.3390/nu11112763
References
- Remely, M.; Stefanska, B.; Lovrecic, L.; Magnet, U.; Haslberger, A.G. Nutriepigenomics: The role of nutrition in epigenetic control of human diseases. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 328–333.
- Blot, W.J.; Tarone, R.E. Doll and Peto’s quantitative estimates of cancer risks: Holding generally true for 35 years. J. Natl. Cancer Inst. 2015, 107, 4.
- Bektas, H.; Karabulut, H.; Doganay, B.; Acar, B. Allergens might trigger migraine attacks. Acta Neurol. Belg. 2017, 117, 91–95.
- Finocchi, C.; Sivori, G. Food as trigger and aggravating factor of migraine. Neurol. Sci. 2012, 33 (Suppl. 1), S77–S80.
- Martin, V.T.; Vij, B. Diet and Headache: Part 1. Headache 2016, 56, 1543–1552.
- Martin, V.T.; Vij, B. Diet and Headache: Part 2. Headache 2016, 56, 1553–1562.
- Owen, L.; Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426.
- Paoli, A.; Moro, T.; Bosco, G.; Bianco, A.; Grimaldi, K.A.; Camporesi, E.; Mangar, D. Effects of n-3 polyunsaturated fatty acids (omega-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar. Drugs 2015, 13, 996–1009.
- Muscogiuri, G.; Palomba, S.; Lagana, A.S.; Orio, F. Current Insights into Inositol Isoforms, Mediterranean and Ketogenic Diets for Polycystic Ovary Syndrome: From Bench to Bedside. Curr. Pharm. Des. 2016, 22, 5554–5557.
- Castaldo, G.; Monaco, L.; Castaldo, L.; Galdo, G.; Cereda, E. An observational study of sequential protein-sparing, very low-calorie ketogenic diet (Oloproteic diet) and hypocaloric Mediterranean-like diet for the treatment of obesity. Int. J. Food Sci. Nutr. 2016, 67, 696–706.
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys) function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532.
- Cioffi, F.; Senese, R.; Lasala, P.; Ziello, A.; Mazzoli, A.; Crescenzo, R.; Liverini, G.; Lanni, A.; Goglia, F.; Iossa, S. Fructose-Rich Diet Affects Mitochondrial DNA Damage and Repair in Rats. Nutrients 2017, 9, 323.
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518.
- Sezgin, Z.; Dincer, Y. Alzheimer’s disease and epigenetic diet. Neurochem. Int. 2014, 78, 105–116.
- Sapienza, C.; Issa, J.P. Diet, Nutrition, and Cancer Epigenetics. Annu. Rev. Nutr. 2016, 36, 665–681.
- Ganesan, A. Multitarget Drugs: An Epigenetic Epiphany. ChemMedChem 2016, 11, 1227–1241.
- Epigenomics Fact Sheet. Available online: (accessed on 10 October 2019).
- Steiner, T.J.; Stovner, L.J.; Birbeck, G.L. Migraine: The seventh disabler. J. Headache Pain 2013, 14, 1.
- Kissoon, N.R.; Cutrer, F.M. Aura and Other Neurologic Dysfunction in or with Migraine. Headache 2017, 57, 1179–1194.
- Seyed Forootan, N.S.; Lee, M.; Guyuron, B. Migraine headache trigger site prevalence analysis of 2590 sites in 1010 patients. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 152–158.
- Levy, D.; Strassman, A.M.; Burstein, R. A critical view on the role of migraine triggers in the genesis of migraine pain. Headache 2009, 49, 953–957.
- Goadsby, P.J.; Lipton, R.B.; Ferrari, M.D. Migraine—current understanding and treatment. N. Engl. J. Med. 2002, 346, 257–270.
- : FDA Approved Drug Products. Available online: (accessed on 10 October 2019).
- Are the New Migraine Medications Working? Available online: (accessed on 10 October 2019).
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia Int. J. Headache 2007, 27, 394–402.
- Vetvik, K.G.; MacGregor, E.A. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017, 16, 76–87.
- Fila, M.; Pawlowska, E.; Blasiak, J. Mitochondria in migraine pathophysiology - does epigenetics play a role? Arch. Med. Sci. AMS 2019, 15, 944–956.
- Sutherland, H.G.; Albury, C.L.; Griffiths, L.R. Advances in genetics of migraine. J. Headache Pain 2019, 20, 72.
- Gormley, P.; Anttila, V.; Winsvold, B.S.; Palta, P.; Esko, T.; Pers, T.H.; Farh, K.H.; Cuenca-Leon, E.; Muona, M.; Furlotte, N.A.; et al. Corrigendum: Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat. Genet. 2016, 48, 856–866.
- de Vries, B.; Frants, R.R.; Ferrari, M.D.; van den Maagdenberg, A.M. Molecular genetics of migraine. Hum. Genet. 2009, 126, 115–132.
- Kocerha, J.; Aggarwal, N. Chapter 8—Epigenetics in Neurobehavioral Disease. In Epigenetics in Human Disease, 2nd ed.; Tollefsbol, T.O., Ed.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Kidlington, UK, 2018; Volume 6, pp. 251–267.
- Majchrzak-Celinska, A.; Baer-Dubowska, W. Pharmacoepigenetics: An element of personalized therapy? Expert Opin. Drug Metab. Toxicol. 2017, 13, 387–398.
- Tomson, T.; Battino, D.; Perucca, E. Valproic acid after five decades of use in epilepsy: Time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016, 15, 210–218.
- Liu, F.; Ma, T.; Che, X.; Wang, Q.; Yu, S. The Efficacy of Venlafaxine, Flunarizine, and Valproic Acid in the Prophylaxis of Vestibular Migraine. Front. Neurol. 2017, 8, 524.
- Eising, E.; Datson, N.A.; van den Maagdenberg, A.M.J.M.; Ferrari, M.D. Epigenetic mechanisms in migraine: A promising avenue? BMC Med. 2013, 11, 26.
- Bishop, K.S.; Ferguson, L.R. The interaction between epigenetics, nutrition and the development of cancer. Nutrients 2015, 7, 922–947.
- Nattagh-Eshtivani, E.; Sani, M.A.; Dahri, M.; Ghalichi, F.; Ghavami, A.; Arjang, P.; Tarighat-Esfanjani, A. The role of nutrients in the pathogenesis and treatment of migraine headaches: Review. Biomed. Pharmacother. 2018, 102, 317–325.
- Barbanti, P.; Fofi, L.; Aurilia, C.; Egeo, G.; Caprio, M. Ketogenic diet in migraine: Rationale, findings and perspectives. Neurol. Sci. 2017, 38 (Suppl. 1), 111–115.
- Ruan, H.B.; Crawford, P.A. Ketone bodies as epigenetic modifiers. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 260–266.
- Andreeva, V.A.; Szabo de Edelenyi, F.; Druesne-Pecollo, N.; Touvier, M.; Hercberg, S.; Galan, P. Macronutrient Intake in Relation to Migraine and Non-Migraine Headaches. Nutrients 2018, 10, 1309.
- Winsvold, B.S.; Palta, P.; Eising, E.; Page, C.M.; van den Maagdenberg, A.M.; Palotie, A.; Zwart, J.A. Epigenetic DNA methylation changes associated with headache chronification: A retrospective case-control study. Cephalalgia Int. J. Headache 2018, 38, 312–322.
- Terlizzi, R.; Bacalini, M.G.; Pirazzini, C.; Giannini, G.; Pierangeli, G.; Garagnani, P.; Franceschi, C.; Cevoli, S.; Cortelli, P. Epigenetic DNA methylation changes in episodic and chronic migraine. Neurol. Sci. 2018, 39 (Suppl. 1), 67–68.
- Gerring, Z.F.; McRae, A.F.; Montgomery, G.W.; Nyholt, D.R. Genome-wide DNA methylation profiling in whole blood reveals epigenetic signatures associated with migraine. BMC Genom. 2018, 19, 69.
- Wan, D.; Hou, L.; Zhang, X.; Han, X.; Chen, M.; Tang, W.; Liu, R.; Dong, Z.; Yu, S. DNA methylation of RAMP1 gene in migraine: An exploratory analysis. J. Headache Pain 2015, 16, 90.
- Labruijere, S.; Stolk, L.; Verbiest, M.; de Vries, R.; Garrelds, I.M.; Eilers, P.H.; Danser, A.H.; Uitterlinden, A.G.; MaassenVanDenBrink, A. Methylation of migraine-related genes in different tissues of the rat. PLoS ONE 2014, 9, e87616.
- Lan, X.; Field, M.S.; Stover, P.J. Cell cycle regulation of folate-mediated one-carbon metabolism. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1426.
- Field, M.S.; Kamynina, E.; Chon, J.; Stover, P.J. Nuclear Folate Metabolism. Annu. Rev. Nutr. 2018, 38, 219–243.
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488.
- Soda, K. Polyamine Metabolism and Gene Methylation in Conjunction with One-Carbon Metabolism. Int. J. Mol. Sci. 2018, 19, 3106.
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859.
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84.
- Capelli, I.; Cianciolo, G.; Gasperoni, L.; Zappulo, F.; Tondolo, F.; Cappuccilli, M.; La Manna, G. Folic Acid and Vitamin B12 Administration in CKD, Why Not? Nutrients 2019, 11, 383.
- Sauer, J.; Mason, J.B.; Choi, S.-W. Too much folate: A risk factor for cancer and cardiovascular disease? Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 30–36.
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet, nutrition and the ageing brain: Current evidence and new directions. Proc. Nutr. Soc. 2018, 77, 152–163.
- Balashova, O.A.; Visina, O.; Borodinsky, L.N. Folate action in nervous system development and disease. Dev. Neurobiol. 2018, 78, 391–402.
- Stover, P.J.; Durga, J.; Field, M.S. Folate nutrition and blood-brain barrier dysfunction. Curr. Opin. Biotechnol. 2017, 44, 146–152.
- Joachim, E.; Goldenberg, N.A.; Bernard, T.J.; Armstrong-Wells, J.; Stabler, S.; Manco-Johnson, M.J. The Methylenetetrahydrofolate Reductase Polymorphism (MTHFR c.677C>T) and Elevated Plasma Homocysteine Levels in a U.S. Pediatric Population with Incident Thromboembolism. Thromb. Res. 2013, 132, 170–174.
- Esse, R.; Barroso, M.; Tavares de Almeida, I.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867.
- Siennicka, A.; Zuchowski, M.; Chelstowski, K.; Cnotliwy, M.; Clark, J.S.; Jastrzebska, M. Homocysteine-Enhanced Proteolytic and Fibrinolytic Processes in Thin Intraluminal Thrombus and Adjacent Wall of Abdominal Aortic Aneurysm: Study In Vitro. Biomed. Res. Int. 2018, 2018, 3205324.
- Mandaviya, P.R.; Joehanes, R.; Brody, J.; Castillo-Fernandez, J.E.; Dekkers, K.F.; Do, A.N.; Graff, M.; Hanninen, I.K.; Tanaka, T.; de Jonge, E.A.L.; et al. Association of dietary folate and vitamin B-12 intake with genome-wide DNA methylation in blood: A large-scale epigenome-wide association analysis in 5841 individuals. Am. J. Clin. Nutr. 2019, 110, 437–450.
- Illingworth, R.S.; Gruenewald-Schneider, U.; De Sousa, D.; Webb, S.; Merusi, C.; Kerr, A.R.; James, K.D.; Smith, C.; Walker, R.; Andrews, R.; et al. Inter-individual variability contrasts with regional homogeneity in the human brain DNA methylome. Nucleic Acids Res. 2015, 43, 732–744.
- Rainero, I.; Vacca, A.; Roveta, F.; Govone, F.; Gai, A.; Rubino, E. Targeting MTHFR for the treatment of migraines. Expert Opin. Ther. Targets 2019, 23, 29–37.
- Liu, R.; Geng, P.; Ma, M.; Yu, S.; Yang, M.; He, M.; Dong, Z.; Zhang, W. MTHFR C677T polymorphism and migraine risk: A meta-analysis. J. Neurol. Sci. 2014, 336, 68–73.
- Menon, S.; Lea, R.A.; Ingle, S.; Sutherland, M.; Wee, S.; Haupt, L.M.; Palmer, M.; Griffiths, L.R. Effects of dietary folate intake on migraine disability and frequency. Headache 2015, 55, 301–309.
- Di Rosa, G.; Attina, S.; Spano, M.; Ingegneri, G.; Sgro, D.L.; Pustorino, G.; Bonsignore, M.; Trapani-Lombardo, V.; Tortorella, G. Efficacy of folic acid in children with migraine, hyperhomocysteinemia and MTHFR polymorphisms. Headache 2007, 47, 1342–1344.
- Lea, R.; Colson, N.; Quinlan, S.; Macmillan, J.; Griffiths, L. The effects of vitamin supplementation and MTHFR (C677T) genotype on homocysteine-lowering and migraine disability. Pharmacogenet. Genom. 2009, 19, 422–428.
- Askari, G.; Nasiri, M.; Mozaffari-Khosravi, H.; Rezaie, M.; Bagheri-Bidakhavidi, M.; Sadeghi, O. The effects of folic acid and pyridoxine supplementation on characteristics of migraine attacks in migraine patients with aura: A double-blind, randomized placebo-controlled, clinical trial. Nutrition 2017, 38, 74–79.
- Sadeghi, O.; Maghsoudi, Z.; Khorvash, F.; Ghiasvand, R.; Askari, G. Assessment of pyridoxine and folate intake in migraine patients. Adv. Biomed. Res. 2016, 5, 47.
- Sutherland, H.G.; Hermile, H.; Sanche, R.; Menon, S.; Lea, R.A.; Haupt, L.M.; Griffiths, L.R. Association study of MTHFD1 coding polymorphisms R134K and R653Q with migraine susceptibility. Headache 2014, 54, 1506–1514.
- Liew, S.C.; Gupta, E.D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 2015, 58, 1–10.
- McNulty, H.; Dowey le, R.C.; Strain, J.J.; Dunne, A.; Ward, M.; Molloy, A.M.; McAnena, L.B.; Hughes, J.P.; Hannon-Fletcher, M.; Scott, J.M. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C->T polymorphism. Circulation 2006, 113, 74–80.
- Isobe, C.; Terayama, Y. A remarkable increase in total homocysteine concentrations in the CSF of migraine patients with aura. Headache 2010, 50, 1561–1569.
- Sadeghi, O.; Maghsoudi, Z.; Askari, G.; Khorvash, F.; Feizi, A. Association between serum levels of homocysteine with characteristics of migraine attacks in migraine with aura. J. Res. Med. Sci. 2014, 19, 1041–1045.
- Ho, P.I.; Ortiz, D.; Rogers, E.; Shea, T.B. Multiple aspects of homocysteine neurotoxicity: Glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 2002, 70, 694–702.
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Res. Med. Sci. 2000, 20, 6920–6926.
- Lippi, G.; Mattiuzzi, C.; Meschi, T.; Cervellin, G.; Borghi, L. Homocysteine and migraine. A narrative review. Clin. Chim. Acta 2014, 433, 5–11.
- Shaik, M.M.; Tan, H.L.; Kamal, M.A.; Gan, S.H. Do folate, vitamins B (6) and B (1)(2) play a role in the pathogenesis of migraine? The role of pharmacoepigenomics. CNS Neurol. Disord. Drug Targets 2014, 13, 828–835.
- Meijers, J.M.; van Bokhorst-de van der Schueren, M.A.; Schols, J.M.; Soeters, P.B.; Halfens, R.J. Defining malnutrition: Mission or mission impossible? Nutrition 2010, 26, 432–440.
