Matricellular proteins harbor functional sites within their molecular structures. These functional sites are released via proteolytic cleavage by inflammatory proteinases, and the peptides containing these hidden functional sites have unique biological activities that are often not detected in the parent molecules. A peptide containing the functional site of tenascin-C (TNC), termed TNIIIA2, which is highly released at sites of inflammation and in the tumor microenvironment, has the ability to potently and persistently activate β1-integrins. Based on these activities, TNIIIA2-containing TNC fragments/peptides are involved in the acquisition of aggressiveness in cancer progression.
- Tenascin-C
- TNIIIA2
- β1-integrin
- integrin activation
- matricellular protein
- platelet-derived growth factor
- matricryptic site
- matricryptin
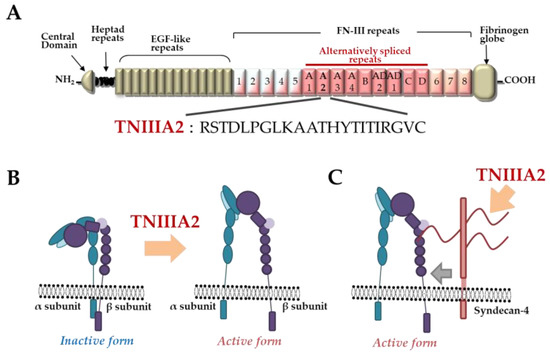
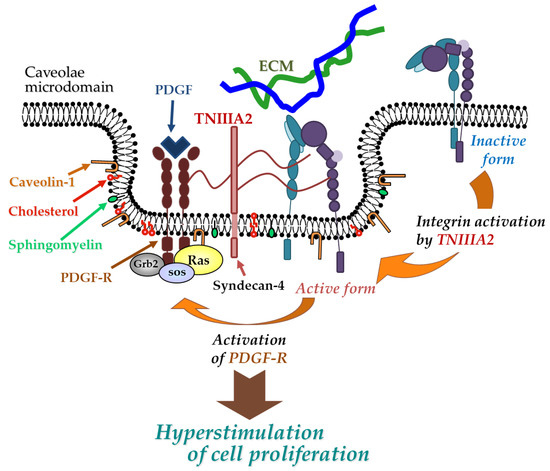
This entry is adapted from the peer-reviewed paper 10.3390/molecules25143239
References
- Sean P Giblin; Kim S Midwood; Tenascin-C: Form versus function. Cell Adhesion & Migration 2014, 9, 48-82, 10.4161/19336918.2014.987587.
- Kim S Midwood; Sandra Sacre; Anna Maria Piccinini; Julia Inglis; Annette Trebaul; Emma Chan; Stefan Drexler; Nidhi Sofat; Masahide Kashiwagi; Gertraud Orend; et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nature Medicine 2009, 15, 774-780, 10.1038/nm.1987.
- Hidenori Suzuki; Masashi Fujimoto; Fumihiro Kawakita; Lei Liu; Fumi Nakano; Hirofumi Nishikawa; Takeshi Okada; Kyoko Imanaka-Yoshida; Toshimichi Yoshida; Masato Shiba; et al. Toll-Like Receptor 4 and Tenascin-C Signaling in Cerebral Vasospasm and Brain Injuries After Subarachnoid Hemorrhage.. Acta Neurochirurgica Supplement 2020, 127, 91-96, 10.1007/978-3-030-04615-6_15.
- Taizo Kimura; Kazuko Tajiri; Akira Sato; Satoshi Sakai; Zheng Wang; Toshimichi Yoshida; Toshimitsu Uede; Michiaki Hiroe; Kazutaka Aonuma; Masaki Ieda; et al. Tenascin-C accelerates adverse ventricular remodelling after myocardial infarction by modulating macrophage polarization. Cardiovascular Research 2018, 115, 614-624, 10.1093/cvr/cvy244.
- Swati Bhattacharyya; Wenxia Wang; Luisa Morales-Nebreda; Gang Feng; Minghua Wu; Xiaodong Zhou; Robert Lafyatis; Jungwha Lee; Monique Hinchcliff; Carol Feghali-Bostwick; et al. Tenascin-C drives persistence of organ fibrosis. Nature Communications 2016, 7, 11703, 10.1038/ncomms11703.
- Kim S. Midwood; Thomas Hussenet; Benoit Langlois; Gertraud Orend; Advances in tenascin-C biology. Cellular and Molecular Life Sciences 2011, 68, 3175-3199, 10.1007/s00018-011-0783-6.
- Joni Leppänen; Ville Lindholm; Joel Isohookana; Kirsi-Maria Haapasaari; Peeter Karihtala; Petri P. Lehenkari; Juha Saarnio; Joonas H Kauppila; Tuomo J. Karttunen; Olli Helminen; et al. Tenascin C, Fibronectin, and Tumor-Stroma Ratio in Pancreatic Ductal Adenocarcinoma. Pancreas 2019, 48, 43-48, 10.1097/mpa.0000000000001195.
- Wenbo Qi; Zhaoting Yang; Haoyue Li; Yan Cui; Yanhua Xuan; The role of Tenascin-C and Twist1 in gastric cancer: cancer progression and prognosis. APMIS 2019, 127, 64-71, 10.1111/apm.12919.
- Tomohiro Murakami; Hirotoshi Kikuchi; Hisato Ishimatsu; Ichirota Iino; Amane Hirotsu; Tomohiro Matsumoto; Yusuke Ozaki; Toshiki Kawabata; Yoshihiro Hiramatsu; Manabu Ohta; et al. Tenascin C in colorectal cancer stroma is a predictive marker for liver metastasis and is a potent target of miR-198 as identified by microRNA analysis. British Journal of Cancer 2017, 117, 1360-1370, 10.1038/bjc.2017.291.
- Zhao-Ting Yang; So-Young Yeo; Yong-Xue Yin; Zhen-Hua Lin; Hak-Min Lee; Yan-Hua Xuan; Yan Cui; Seok-Hyung Kim; Tenascin-C, a Prognostic Determinant of Esophageal Squamous Cell Carcinoma. PLOS ONE 2016, 11, e0145807, 10.1371/journal.pone.0145807.
- Vasilena Gocheva; Alexandra Naba; Arjun Bhutkar; Talia Guardia; Kathryn M. Miller; Carman Man-Chung Li; Talya L. Dayton; Francisco J. Sánchez-Rivera; Caroline Kim-Kiselak; Noor Jailkhani; et al. Quantitative proteomics identify Tenascin-C as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival.. Proceedings of the National Academy of Sciences 2017, 114, E5625-E5634, 10.1073/pnas.1707054114.
- A Ishihara; T Yoshida; H Tamaki; T Sakakura; Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance.. Clinical Cancer Research 1995, 1, 1035-41, .
- J. Qi; Darian R. Esfahani; T. Huang; Patrick A. Ozark; Elizabeth T. Bartom; Rintaro Hashizume; Erin R. Bonner; S. An; Craig Horbinski; C. David James; et al. Tenascin-C expression contributes to pediatric brainstem glioma tumor phenotype and represents a novel biomarker of disease. Acta Neuropathologica Communications 2019, 7, 75, 10.1186/s40478-019-0727-1.
- Anna Mary Steitz; Alina Steffes; Florian Finkernagel; Annika Unger; Leah Sommerfeld; Julia M. Jansen; Uwe Wagner; Johannes Graumann; Rolf Müller; Silke Reinartz; et al. Tumor-associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (TGFBI) and tenascin C. Cell Death & Disease 2020, 11, 1-15, 10.1038/s41419-020-2438-8.
- Pablo Igor Ribeiro Franco; Arthur Perillo Rodrigues; Liliana Borges De Menezes; Marina Pacheco Miguel; Pablo Igor Ribeiro Francoa; Liliana Borges De Menezes Leite; Tumor microenvironment components: Allies of cancer progression. Pathology - Research and Practice 2020, 216, 152729, 10.1016/j.prp.2019.152729.
- Kim S. Midwood; Matthias Chiquet; Richard P. Tucker; Gertraud Orend; Tenascin-C at a glance. Journal of Cell Science 2016, 129, 4321-4327, 10.1242/jcs.190546.
- Margret Dueck; Stefan Riedl; Ulf Hinz; Andrea Tandara; Christian Herfarth; Andreas Faissner; Detection of tenascin-C isoforms in colorectal mucosa, ulcerative colitis, carcinomas and liver metastases. International Journal of Cancer 1999, 82, 477-483, 10.1002/(sici)1097-0215(19990812)82:4<477::aid-ijc2>3.0.co;2-5.
- Yohei Saito; Hisae Imazeki; Shogo Miura; Tomohisa Yoshimura; Hiroaki Okutsu; Yosei Harada; Toshiyuki Ohwaki; Osamu Nagao; Sadahiro Kamiya; Ryo Hayashi; et al. A Peptide Derived from Tenascin-C Induces β1 Integrin Activation through Syndecan-4. Journal of Biological Chemistry 2007, 282, 34929-34937, 10.1074/jbc.m705608200.
- Rika Tanaka; Yutaka Seki; Yohei Saito; Sadahiro Kamiya; Motomichi Fujita; Hiroaki Okutsu; Takuya Iyoda; Tatsuya Takai; Toshiyuki Owaki; Hirofumi Yajima; et al. Tenascin-C-derived Peptide TNIIIA2 Highly Enhances Cell Survival and Platelet-derived Growth Factor (PDGF)-dependent Cell Proliferation through Potentiated and Sustained Activation of Integrin α5β1. Journal of Biological Chemistry 2014, 289, 17699-17708, 10.1074/jbc.m113.546622.
- Rika Tanaka; Toshiyuki Owaki; Sadahiro Kamiya; Takuya Matsunaga; Kazuya Shimoda; Hiroaki Kodama; Ryo Hayashi; Takashi Abe; Yosei P. Harada; Motoyuki Shimonaka; et al. VLA-5-mediated Adhesion to Fibronectin Accelerates Hemin-stimulated Erythroid Differentiation of K562 Cells through Induction of VLA-4 Expression. Journal of Biological Chemistry 2009, 284, 19817-19825, 10.1074/jbc.m109.009860.
- Yohei Saito; Toshiyuki Owaki; Takuya Matsunaga; Mizue Saze; Shogo Miura; Mao Maeda; Mayu Eguchi; Rika Tanaka; Junichi Taira; Hiroaki Kodama; et al. Apoptotic Death of Hematopoietic Tumor Cells through Potentiated and Sustained Adhesion to Fibronectin via VLA-4. Journal of Biological Chemistry 2009, 285, 7006-7015, 10.1074/jbc.m109.027581.
- Kazuki Otsuka; Manabu Sasada; Takuya Iyoda; Yusuke Nohara; Shunsuke Sakai; Tatsufumi Asayama; Yusuke Suenaga; Sana Yokoi; Yoshikazu Higami; Hiroaki Kodama; et al. Combining peptide TNIIIA2 with all-trans retinoic acid accelerates N-Myc protein degradation and neuronal differentiation in MYCN-amplified neuroblastoma cells.. American journal of cancer research 2019, 9, 434-448, .
- Kazuki Otsuka; Manabu Sasada; Y U Hirano; Yusuke Nohara; Takuya Iyoda; Yoshikazu Higami; Hiroaki Kodama; Fumio Fukai; Acyclic Retinoid Combined With Tenascin-C-derived Peptide Reduces the Malignant Phenotype of Neuroblastoma Cells Through N-Myc Degradation.. Anticancer Research 2019, 39, 3487-3492, 10.21873/anticanres.13494.
- Motomichi Fujita; Tetsuya Yamamoto; Takuya Iyoda; Tatsuya Fujisawa; Manabu Sasada; Reo Nagai; Chikako Kudo; Kazuki Otsuka; Sadahiro Kamiya; Hiroaki Kodama; et al. Aggressive Progression in Glioblastoma Cells through Potentiated Activation of Integrin α5β1 by the Tenascin-C-Derived Peptide TNIIIA2.. Molecular Cancer Therapeutics 2019, 18, 1649-1658, 10.1158/1535-7163.MCT-18-1251.
- Motomichi Fujita; Tetsuya Yamamoto; Takuya Iyoda; Tatsuya Fujisawa; Reo Nagai; Chikako Kudo; Manabu Sasada; Hiroaki Kodama; Fumio Fukai; Autocrine Production of PDGF Stimulated by the Tenascin-C-Derived Peptide TNIIIA2 Induces Hyper-Proliferation in Glioblastoma Cells.. International Journal of Molecular Sciences 2019, 20, 3183, 10.3390/ijms20133183.
- Motomichi Fujita; Yuka Ito-Fujita; Takuya Iyoda; Manabu Sasada; Yuko Okada; Kazuma Ishibashi; Takuro Osawa; Hiroaki Kodama; Fumio Fukai; Hideo Suzuki; et al. Peptide TNIIIA2 Derived from Tenascin-C Contributes to Malignant Progression in Colitis-Associated Colorectal Cancer via β1-Integrin Activation in Fibroblasts.. International Journal of Molecular Sciences 2019, 20, 2752, 10.3390/ijms20112752.
- Hideo Suzuki; Manabu Sasada; Sadahiro Kamiya; Yuka Ito; Hikaru Watanabe; Yuko Okada; Kazuma Ishibashi; Takuya Iyoda; Akinori Yanaka; Fumio Fukai; et al. The Promoting Effect of the Extracellular Matrix Peptide TNIIIA2 Derived from Tenascin-C in Colon Cancer Cell Infiltration. International Journal of Molecular Sciences 2017, 18, 181, 10.3390/ijms18010181.
- Motomichi Fujita; Manabu Sasada; Takuya Iyoda; Fumio Fukai; Involvement of Integrin-Activating Peptides Derived from Tenascin-C in Cancer Aggression and New Anticancer Strategy Using the Fibronectin-Derived Integrin-Inactivating Peptide. Molecules 2020, 25, 3239, 10.3390/molecules25143239.
