Aggregation-induced emission (AIE) compounds display a photophysical phenomenon in
which the aggregate state exhibits stronger emission than the isolated units. The common term of
“AIEgens” was coined to describe compounds undergoing the AIE effect. Due to the recent interest
in AIEgens, the search for novel hybrid organic–inorganic compounds with unique luminescence
properties in the aggregate phase is a relevant goal. In this perspective, the abundant, inexpensive,
and nontoxic d10 zinc cation offers unique opportunities for building AIE active fluorophores, sensing
probes, and bioimaging tools. Considering the novelty of the topic, relevant examples collected in
the last 5 years (2016–2021) through scientific production can be considered fully representative of
the state-of-the-art. Starting from the simple phenomenological approach and considering different
typological and chemical units and structures, we focused on zinc-based AIEgens offering synthetic
novelty, research completeness, and relevant applications. A special section was devoted to Zn(II)-
based AIEgens for living cell imaging as the novel technological frontier in biology and medicine.
- AIE
- zinc complex
- fluorescence
1. Introduction
A luminescent material is a material able to emit light in the process of returning from the electronic or vibrational excited state to the ground state after being excited by external energy. The term “luminescence” was introduced in 1888 by the physicist and historian Eilhard Wiedemann to describe light emission not simply related to an increase in temperature. Photo-induced luminescence or photoluminescence (PL), often referred simply as “luminescence”, is the light emission in the optical range of visible, ultraviolet, or infrared light. According to the mode of excitation and relaxation, luminescence can be classified into various types, including fluorescence and phosphorescence.
Contrary to phosphorescence, fluorescence is a form of photoluminescence in which the excitation–emission process occurs very quickly [1][2]. After a molecule absorbs energy from a light source and becomes excited, fluorescence occurs within nanoseconds. Upon excitation with electromagnetic energy at the correct wavelength, an electron in the fluorescent molecule is promoted to an upper level, and finally, the energy is released in the form of a photon (fluorescence emission) while the electron moves back down to the lower energy level. In most cases, fluorescence requires a longer excitation wavelength—and so lower energy—than the absorbed radiation.
Since 1800, fluorescent organic dyes highly emissive in diluted solutions were examined. In the middle of the nineteenth century, the chemist Adolf von Baeyer developed fluorescein as a synthetic organic material highly fluorescent in aqueous solutions in daylight. This fluorescence quenching effect at high concentrations was clarified in 1955 by Th. In 1970, the ACQ effect was told as “common to most aromatic hydrocarbons and their derivatives” by J. B. Birks, in his book entitledPhotophysics of Aromatic Molecules[3].
With the rise and development of new technologies, the twentieth century opened the doors to a new perspective about luminescent materials. In most modern applications, such as optoelectronic devices (as laser, optical storage, and OLEDs) and biomedical tools, fluorescence materials are used as solids and/or aggregates [4][5][6][7][8][9][10][11][12]. The need for materials suitable for such applications led to a growing interest in the design and synthesis of aggregate emitters.
The first pivotal report containing the concept of aggregation-induced emission materials is due to Tang and coworkers [13]. Aggregation-induced emission (AIE) compounds display a photophysical phenomenon in which molecular aggregates exhibit stronger emission than single molecules. non-emissive in solutions up to 80% water fraction. Above this threshold, strong fluorescence was observed due to the strong aggregation of the molecules.
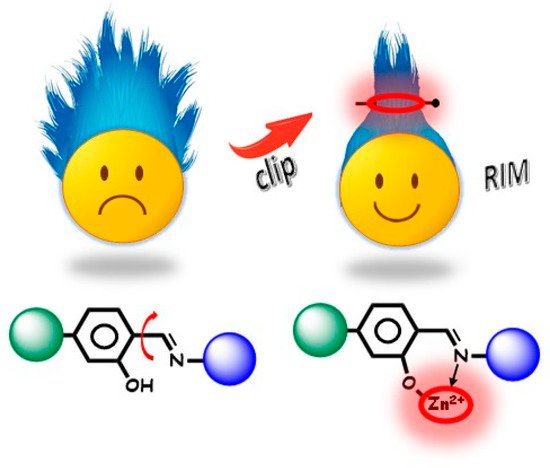
Milestones for AIE theoretical development are several scientific publications from 2001 up to now. In 2003, again Tang and coworkers proposed the restriction of intramolecular rotations (RIR) mechanism based on the study of hexaphenylsilole [14] and proved it as applicable to most AIEgens. Contrarily, upon aggregation, the restriction of the intramolecular motions suppresses the radiationless decay pathway. A generalised mechanism for AIEgens named “RIM” (restriction of intramolecular motions) was introduced in 2014 by combining RIR and RIV effects, so including rotation, vibration, bending, flapping, twisting, and other intramolecular motions concurring to the radiationless decay pathways [15][16][17][18][19].
Specifically, to predict AIEgens behaviour, other energetic parameters are involved and must be considered. The Duschinsky rotation energy [20][21][22][23][24][25] (due to the difference between the ground state and excited-state potential energy surfaces, calculated by the harmonic oscillator model); the reorganisation energy [26] (required to relax the structure and environment upon electron transfer); the formation of J-aggregates (causing a bathochromic shift in the absorption due to π–π stacking of the aromatic moieties) [27]. Finally, the restricted access to a conical intersection (RACI) model to analyse the global potential energy surface topology was introduced in 2013 by Blancafort and coworkers [25]. Conical intersections were described as regions of the potential energy surface where the ground and excited states are degenerate, and the probability of non-radiative internal conversion is maximal.
To conclude, a correct theoretical setting of the chemical system is crucial for the actual prediction of the photoluminescence yield of the AIE fluorescent dye. The observation of the effects involved in the activation of emission in the aggregate state gave scientists new ideas about AIEgens design.
The great potential of AIEgens is documented by the number of publications and citations about the topic, exponentially increasing since the first AIE report in 2001. To date, more than 2000 papers (articles and many reviews) have been published under the keyword “AIEgen”, and AIE topic was ranked no. In the last 20 years, AIEgens have been deeply explored and their unique advantages over conventional ACQ molecules fully recognised. The implementation in AIEgens study is moved by the cutting-edge technologies demand, ranging from optoelectronic to sensing and bioimaging [28].
Many optoelectronic techniques greedily require emissive solid layers. The earliest application of AIEgen in optoelectronic devices was reported in 2001 as blue-emissive AIE siloles with excellent external quantum efficiency [29]. Several AIEgens have been designed with the precise aim of obtaining solid-state emissions. In addition, the whole emission colour display, up to the NIR region, can be easily obtained by structural modifications in the AIEgen skeleton.
Due to their versatility, AIEgens can be easily obtained in a polymeric- or macro-structured form by reaction of functionalised fluorophores or by doping the fluorophores in a polymeric matrix. Macromolecular materials are ideal candidates for optoelectronic applications based on solid-state layers, such as organic light-emitting diodes (OLEDs, optical waveguides, and luminescent solar concentrators, or even soft-matter-based devices such as light-emitting electrochemical cells (LECs) and liquid-crystal displays [30][31][32][33][34].
Other significant applications of AIEgens are fluorescence chemosensors and multiple-stimuli (such as pH, electromagnetic radiation, morphology) responsive materials [35][36][37][38]. AIEgens can be utilised as fluorescent indicators/markers to characterise supramolecular interactions and macromolecular motions in the solid state, in an aggregate gel phase or even in a water-concentrated solution. In 2005, Tang and coworkers produced chemosensors based on silole as sensors for the regioisomers of nitroanilines [39] and in 2010, Park and coworkers promoted AIEgens as stimuli–responsive materials. To date, several AIE-based chemosensors were reported for the detection of ions, metals, small organic molecules, and biological targets [40][41][42][43][44][45][46][47][48].
Porous crystalline solids such as inorganic nanoparticles (NPs), metal–organic frameworks (MOFs), covalent organic frameworks (COFs), and carbon nanotubes (CNTs) are materials where the choice of suitable AIE active fragments can produce highly engineered composite materials with unique properties [41][42][43]. In the composite material, the AIE moieties interact with a regular structure resulting in tunable electronic and optical properties. Low self-quenching and a highly modulable inner chemical environment can be achieved. Thanks to the porous architecture, such nanostructured AIEgens are promising candidates for fluorescence sensing with high sensitivity and selectivity toward specific analytes [43][44][45][46][47][48].
The incorporation of AIEgens in bio-compatible materials through various methods (covalent or coordinate bonds, noncovalent interactions) provides AIEgen-based composite biomaterials for several uses. In 2012, Tang and Liu and coworkers developed AIEgen bioprobes for cell-imaging and monitoring of biological processes [46]. Due to their permeability and retention, biocompatible AIE-based dots and NPs were also applied for in vivo cancer imaging and diagnostics [47][48]. Again, Tang and coworkers, in 2018, reported a simple approach to link AIEgens to bio-relevant species for biological labelling and monitoring [49].
Lessons learned from these advanced tools provide new ways of thinking about the most relevant in vivo applications.
In the following years, many purely organic, aromatic and heteroaromatic RIM undergoing AIEgens were described. Specifically, AIEgens bearing cores such as tetraphenylethene, thiophene-triphenylamine, tetraphenylpyrazine, quinoline, 9,10-distrylanthracene, dithiole, and derivatives have been extensively reviewed in recent articles [50][51][45][52][53][54][55][56][57][58].
Despite the obvious advantages of organic fluorophores, which are singlet emitters, heavy atoms such as transition metals display triplet emission. The related spin–orbit coupling leads to efficient singlet–triplet state mixing so that the presence of a heavy atom can improve the photophysical properties of π-conjugated ligands [59][60][61][62]. The formation of metal complexes represents a strategy for obtaining new luminescent materials with long luminescence lifetimes, large Stoke’s shifts, and high PLQYs in the visible region. Metal complexes are potentially unique luminophores with highly tunable structures and photophysics.
The origin of the AIE effect in transition metal complexes can be explained based on the competing radiative and non-radiative de-excitation paths. Besides, blocking the non-radiative deactivation pathway, the presence of the coordinated metal can also produce a more efficient emitting state of the organic part in its aggregate state. In fact, the electronic charge in the complex can be transferred between different molecular moieties, involving electron transfer between metal cation and ligands. The AIE effect is potentially ascribable to simultaneous changes of the emitting state and activation of RIM and/or RACI mechanisms.
Several transition metals such as Ir(III), Pt(II), Re(I), Ru(II), Os(IV), Au(I), and Pd(II) are reported to cause the activation of the AIE channel [62]. The development of AIE-active complexes seems to be a promising strategy for many AIE systems where the metal is strictly involved in the emission process. In all cases, ML and/or LM transfer must be expected in the emissive complexes.
In the growing demand for high-performance devices, sustainability is a relevant parameter. Zinc, as a small eco-friendly cation, is a good alternative to other hazardous or expansive metals. Specifically, zinc (II) complexes exhibit fluorescence intensity and/or colour tuning in dependence on the electronic pattern of the ligands and the coordination pattern imposed by the ligands. In fact, in the d10closed-shell zinc (II) ion, d–d electronic transitions are not expected.
In most cases, the cation acts as a constraint for the ligand by locking it into a favourable emissive conformation, with few electronic effects mostly due to π–π* LCT transitions. Chelation enhanced fluorescence (CHEF mechanism) is often involved in zinc (II) binding as the result of the stabilisation of the excited state in poorly emissive ligands [7]. Therefore, the role of zinc (II) cation can be to turn a non-emissive ligand in an AIEgen molecule upon coordination. In addition to the “optically innocent clip role”, zinc cation offers the ability to give rise to the most varied architectures through coordination of organic ligands, self-assembly of two and three-dimensional macrostructures, aggregation of nanomaterials, and production of regular or amorphous structures [63][64][65][66][67][68][69].
Despite several studies encompassing the topic of AIEgens, in this review, we will focus our attention on the role of Zn(II) in the design of AIE active complexes and materials. Our main aim is to give a unified overview of AIEgens involving zinc (II) cation (abbreviated as Zn AIEgens). With the term “Zn AIEgens”, we mean: the zinc (II) complexes exhibiting AIE properties; the AIEgens giving a fluorescence response in the presence of zinc (II) cation; the zinc (II) complexes exploiting an AIE mechanism to detect specific analytes. In short, with this review, we wanted to answer the question: what zinc (II) is dealing with AIEgens.
Considering the novelty of the topic, we cut the last 5 years (years 2016–2021) as representative of the recent scientific production. Selected examples were reported to elucidate the state-of-the-art, starting from the simple phenomenological approach and considering different typological and chemical units and structures. We will focus on the recent Zn AIEgens based on the synthetic novelty, research completeness, fluorescence response, theoretical deepening, and relevant applications. Newly developed Zn AIEgens;Zn AIEgens for OLEDs and other optical technologies;The role of zinc (II) in AIEgen chemosensing;Zn AIEgens for cell imaging.
Scientific literature, including reviews, were consulted to understand the structure, the optical response, and the operating mode of Zn AIEgens. Finally, we underlined the representative applications for the targeted biological and medical scope. By use of a quick glance representation which should be considered an integral part of the discussion, we propose an intuitive overview of groups of structures examined according to the application area.
2. Newly Developed Zn AIEgens
The starting point is represented by the design and synthesis of simple small organic molecules, even non-emissive, acting toward Zn(II) as ligands or chelators. The zinc complexes are expected to meet specific requirements, such as high PLQYs in the aggregate phase, photostability, large lifetime, and Stokes shifts. An easy test to assess the AIE nature of the compound consists of recording emission intensity as the ratio of non-solvent/solvent increases [70]. Emission is expected to grow as the aggregation increases.
The synthetic Zn AIEgens reported in this section are referred to as “potentially useful” materials for optoelectronic applications and/or optical techniques. On the other hand, articles including targeted applications will be reported inSection 3.
InSection 2.1, we will report the most relevant examples of novel Zn AIEgens obtained by encumbered nitrogen and/or oxygen donor-based low-molecular-weight ligands (RIM effect undergoing complexes). Our discussion will be articulated based on structural similarities and differences and on the AIE activated channel. The related structures are summarised inFigure 1, accordingly with discussion. InSection 2.2, we referred to a few relevant examples of metal–organic frameworks acting as highly fluorescent Zn AIEgens.
The most common and easy approach to obtain Zn AIEgens is to enhance steric hindrance by adding bulky substituents to the chelating ligands. The ligand undergoes a conformational block upon coordination, producing the RIM fragment. A selection of relevant examples of synthetic Zn AIEgens undergoing the RIM effect is grouped based on chemical/structural features. As it will be discussed, some peculiar skeletons able to cause the RIM effect are recurrent.
In solution, PLQYs increased as the solution turned into aggregate state PLQYs by adding a non-solvent. In the solid-state, each complex showed different emission wavelengths and spectra depending on the crystal packing structure. With respect to the solution behaviour, the different PLQYs and lifetimes recorded on the crystalline complexes proved the activation of different photophysics in the solid state. As a result, the complex obtained by reacting the composed ligand with zinc (II) cation is a Zn AIEgen and exhibits reversible fluorescence colour and intensity changes
Commonsalentype ligands with encumbered substituents are excellent candidates in building Zn AIEgens, zinc cation acting as a constraint of the structure. Upon irradiation with UV light, the N=N azo bridge exhibitscis–transisomerisation both in the ligands and in their zinc (II) complexes. Comparative photoswitching studies and DFT analysis was performed on ligands and complexes based on X-ray analysis. TD-DFT analysis was employed to explore the role of the zinc atom in the emission mechanism, resulting in a structural change of the ligand from the lactam form (3,4-dihydro-3-oxo-2-quinoxalinecarboxylic acid) to the enol form (3-hydroxy-2-quinoxalinecarboxylic acid) in the presence of zinc (II).
A systematic study of isomeric Schiff-base zinc complexes showing relevant differences in their crystalline pattern and emission behaviour was published in 2018 by Rosita Diana and coworkers [71]. The reactions of the ligands with zinc (II) acetate in pyridine gave crystalline complexes with the general formula Zn(L)2py2. The reaction with zinc cation self-assemblies themetaderivative ligand in a stable glassy network, with Zn(L)2general stoichiometry and PLQY = 44%. and acylhydrazones ligands acting as differently substitutedO,
The results achieved in both articles suggested that the polymeric or reticulated macrostructures obtained by zinc-driven self-assembly provide emission enhancement. Both in the case of an amorphous and crystalline material, the tight structure with optically “innocent” zinc nodes could guarantee a more efficient electron hopping in the whole macrostructure.
The role of the metal in assembly together non-fluorescent components was examined in 2020 by Jubaraj B. Baruah and coworkers [72]. The frontier molecular energy levels of different combinations of the positional isomeric complexes were ascertained. As expected, the participation of water molecules to form aggregates with the complexes caused increased emission intensity up to a characteristic limit. Above that concentration, emission quenching was recorded due to the equilibrium between the complex and the hydrate cation.
Other structures causing the AIE effect by steric constrain are metallo–supramolecular architectures, achieved by zinc-driven self-assembling reaction. Specifically, emissive or non-emissive polyfunctional ligands can be assembly in macrocycles or metallocycles by reaction with zinc cation. By gradual addition of a non-solvent to the complexes dissolved in chloroform, a visible aggregation was induced, and emission enhancement was recorded. The complexes showed yellow to orange–red emission in the solid state (PLQE = 1–5%), displaying up to a 75-fold increase in peak emission intensity upon aggregation, in the best case.
Owing to the strong chelating ability, TPY derivatives can form stable complexes with several different transition metal ions. Xiaopeng Li and coworkers in 2019 [73] studied self-assembly of emissive metallocycles with tetraphenylethylene (TPE), boron-dipyrromethene (BODIPY), and terpyridine (TPY). Combining the three blocks into one system by zinc coordination driven self-assembly reaction, a metallocycle dimer with typical AIE characteristics was produced. Interestingly, by combining the three fluorophores into a metallo–supramolecular architecture, emissive properties were recorded both in solution and in the aggregated state.
Metal-organic frameworks (MOFs) are hybrid organic–inorganic crystalline materials derived by the regular array of metal cations (or clusters) surrounded by organic linkers. Professor Omar Yaghi at UC Berkeley in the late 1990s (“Design and synthesis of an exceptionally stable and highly porous metal-organic framework”) presented MOFs for the first time, and they rapidly become a cutting-edge research field. The synergistic effects of structures and compositions make MOFs fascinating examples of unique structural design and tunable properties. Different metal atoms and organic linkers lead to MOFs selectively absorbing targeted molecules /ions.
In recent decades, MOFs with luminescence characteristics (luminescent MOFs, LMOFs) has been considered as the new pathway for the fabrication of solid-state luminescent nanomaterials. AIEgen ligands can be employed as building blocks in the MOFs construction. To retain the fluorescence of AIE linkers or activate the AIE channel, the closed-shell zinc (II) ion with low-lying d-orbital energy can be preferred to classic heavy metals. nodes afford MOFs with strong luminescence due to constraining effect on the AIE ligands.
In this section, we reported a few relevant examples of LMOFs containing zinc (II) and acting as highly active AIEgens (we will use the term “AIE Zn-MOFs”). In Figure 2, we reported a schematic representation focusing on the structural feature.
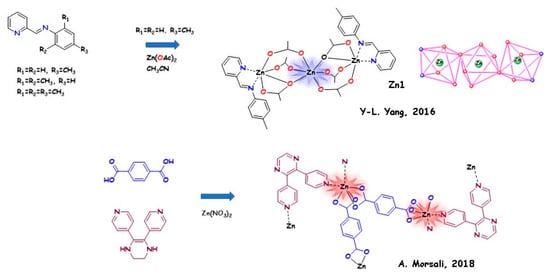
In 2016, Yu-Lin Yang and coworkers [74] used five Schiff-base ligands containing aN-(pyridine-2-yl) fragment with different alkyl substitutions on the phenyl ring for the synthesis of Zn(II)/Cd(II) complexes. The structures of the ligands can direct the formation of 3D supramolecular LMOFs due to hydrogen bonds and π–π interactions. Nine Zn(II)/Cd(II) complexes were obtained, displaying deep blue emissions (401−436 nm) in acetonitrile solution and light blue/bluish green emissions (485−575 nm) in the solid state. Structural analysis gave information on the crystalline packing of the complexes.
Ali Morsali and coworkers [75] in 2018 synthesised a turn-on AIE-based MOF by in situ ligand fabrication and employing different valence-shell cations, with the aim of investigating the effect of metal nodes. The coordination of 5,6-di(pyridin-4-yl)-1,2,3,4-tetrahydropyrazine (AIE fluorophore) and terephthalic acid ligand with Zn(II), Co(II), or Cd(II) leads to the preparation of strongly luminescent MOFs (named “TMU-40(Zn)”, “TMU-40(Cd)”, and “TMU-40(Co)”). The formation of rigid frameworks improved the fluorescence of the organic parts more with zinc than with the other two metals (PLQY = 38.2% with respect to 31.17% and 11.69%, respectively).
3. Zn AIEgens for OLEDs and Other Optical Technologies
Solid-state emitters have a great potential for the development of novel optoelectronic technologies, such as OLEDs and other optical tools. Zinc (II) complexes are a potential alternative to expensive transition metals complexes of iridium, osmium, and platinum, and can represent excellent luminescent and electron-accepting/transporting materials. They can be employed as emitter layers in a variety of forms: solid powders, dyes dispersed in host solid and/or gel matrixes, and metallo–polymers. Since the first OLED based on a Schiff-base zinc (II) complex developed by Hamada in 1993 [76], the research on highly efficient emissive zinc (II) complexes continued.
In this section, we will discuss the most relevant examples of opto-applications of Zn AIEgens, from OLEDs and LECs to other cutting-edge technologies. In Figure 3, we collected the related structures, referring to the most PL active architectures.
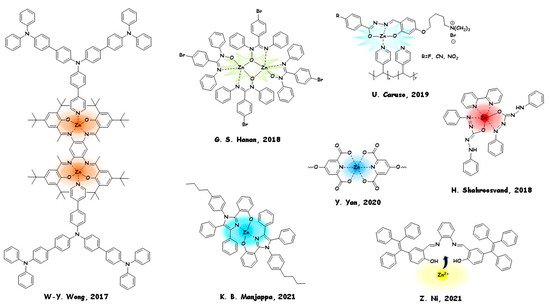
The electroluminescence (EL) properties achieved by zinc (II) complexes were checked: peak luminance (Lmax) of 3589 cd m−2, maximal external quantum efficiency (ηext) of 1.46%, maximum current efficiency (ηL) of 4.1 cd A−1, and maximal power efficiency (ηP) of 3.8 lm W−1. Garry S. Hanan and coworkers in 2018 [77] synthesised a dinuclear complex of zinc (II) with 4-bromo-N,N′-diphenylbenzamidinate N-oxide. This compound is a Zn AIEgen and was used as a dopant in a co-host matrix of the emissive layer used in the fabrication of a solution-processed white–green WOLED. A luminance efficiency and power efficiency of 1.12 cd/A and 0.30 lm/W, respectively, were obtained.
RGB (red, green, blue) metallopolymer emitters with potential in WOLEDs preparation were examined by Ugo Caruso and coworkers in 2019 [54]. ,N,Otridentate ligands with a different electron-withdrawing substituent and a charged moiety were prepared by grafting ligand-Zn(II) coordination fragments onto commercial poly-(4-vinylpyridine). A rare example of LEC was prepared by Hashem Shahroosvand and coworkers in 2018 [78], based on a blend of the cationic complex [Ru(bpy)3]2+and a neutral zinc (II) complex derived from diphenylcarbazone ligands. The crystal structure of the Zn fluorescent complex was examined, and the assignment of ground- and excited-state transitions was achieved by TD-DFT analysis.
First, it was used for the fabrication of an OLED, with a maximum brightness of 15,000 cd/m2(16 V), and external quantum efficiency close to 3.8% (comparable with state-of-art of the purely fluorescent non-thermally activated OLEDs). Fingerprint detection is a crucial area in forensic science and is based on fluorescent dyes. Moved by a similar approach, Yun Yan and coworkers in 2020 [79] reported a water-based polyion anticounterfeiting micellar ink displaying full-spectral RGB emission colours by a combination of AIEgens and luminescence of rare earth metals. Blue emission was achieved from the AIE fluorophore tetraphenylethylene (TPE) linked by zinc cation into coordination supramolecular polymers.
4. Zn AIEgens for Cell Imaging
The scientific research about sensitive and selective fluorescent probes opens new horizons for biological parameters detection. By modern microscopy and imaging techniques in combination with fluorescence sensor, sharper cytologic details can be visualised and recorded. From the first fluorescence microscope (due to Heimstadt in 1911 and Lehmann in 1913) to modern bioimaging techniques, more than a century has passed, and research made great strides from the early fluorophores (as rhodamine, fluorescein, flavin derivatives) to the modern highly engineered fluorogenic dyes. Nonetheless, the search for new marking systems is constantly evolving.
Time-resolved imaging is a modern microscopy technique whereby fast kinetic and PL decay parameters (decay times and the corresponding resolved amplitudes) are directly and simultaneously measured throughout an image in an optical microscope. Thus far, most fluorescent dyes utilised in sensing commonly suffer from severe concentration or the ACQ effect, and their fluorescence lifetimes can be shortened to nanoseconds in the aggregate phase. This behaviour is unwelcomed for the time-resolved fluorescence imaging technique. The employ of AIE active sensors or markers can solve the ACQ problems due to the high fluorescence of the aggregates in aqueous media and short fluorescence lifetimes.
In this context, Zn AIEgens have drawn considerable attention, thanks to the synthetic feasibility, tuneable PL pattern, and biocompatibility.
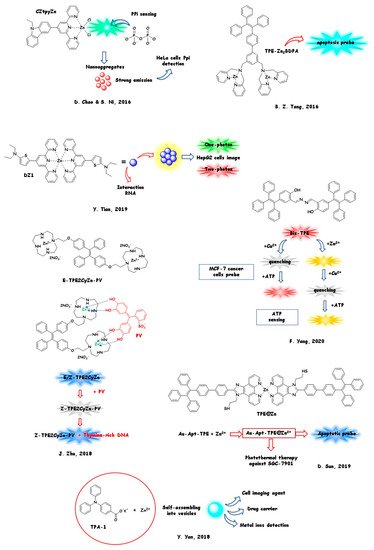
Again, the involvement of zinc (II) cation with nanosized AIE sensors can be as a biologically relevant analyte or as a part of the sensor itself. In the next section (Section 5.1), we will discuss the monitoring of the zinc (II) level in living biological substrates. The structures and the sensing/marking mechanism are reported in Figure 4.
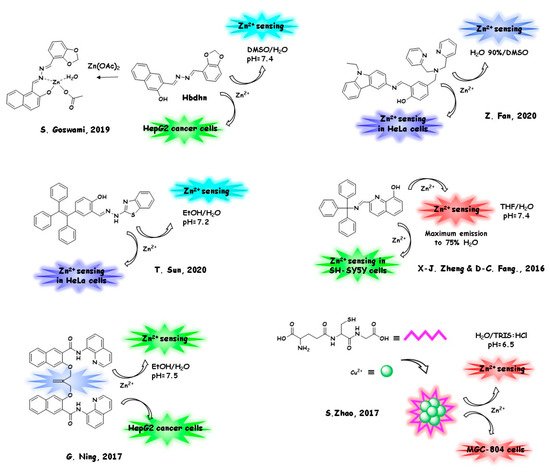
In Section 5.2, we will report a selection of the most relevant biosensors and markers containing zinc in their architecture and employed for their sensing/marking ability in living cells (Figure 5). The latter bio-probes can be considered as a class of innovative and desirable biocompatible tools, with a strong technological impact on diagnostic imaging methods.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26144176
References
- Langhals, H. Fluorescence and fluorescent dyes. Phys. Sci. Rev. 2020, 5, 1–26.
- Valeur, B.; Berberan-santos, N. Emergence of Quantum Theory. J. Chem. Educ. 2011, 88, 731–738.
- Birks, J.B. Photophysics of Aromatic Molecules; Wiley-Interscience: London, UK, 1970; ISBN 0471074209 9780471074205.
- Dumur, F. Zinc complexes in OLEDs: An overview. Synth. Met. 2014, 195, 241–251.
- Diana, R.; Panunzi, B.; Tuzi, A.; Piotto, S.; Concilio, S.; Caruso, U. An Amphiphilic Pyridinoyl-hydrazone Probe for Colorimetric and Fluorescence pH Sensing. Molecules 2019, 24, 3833.
- Huang, J.; Nie, H.; Zeng, J.; Zhuang, Z.; Gan, S.; Cai, Y.; Guo, J.; Su, S.-J.; Zhao, Z.; Tang, B.Z. Highly Efficient Nondoped OLEDs with Negligible Efficiency Roll-Off Fabricated from Aggregation-Induced Delayed Fluorescence Luminogens. Angew. Chem. Int. Ed. 2017, 56, 12971–12976.
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238.
- Wang, Z.; Wang, R.; Mi, Y.; Lu, K.; Liu, Y.; Yang, C.; Zhang, J.; Liu, X.; Wang, Y.; Shuai, Z.; et al. Creating Side Transport Pathways in Organic Solar Cells by Introducing Delayed Fluorescence Molecules. Chem. Mater. 2021, 33, 4578–4585.
- De Girolamo Del Mauro, A.; Diana, R.; Grimaldi, I.A.; Loffredo, F.; Morvillo, P.; Villani, F.; Minarini, C. Polymer solar cells with inkjet-printed doped-PEDOT: PSS anode. Polym. Compos. 2013, 34, 1493–1499.
- Lee, H.L.; Lee, K.H.; Lee, J.Y.; Lee, H.J. Molecular design opening two emission pathways for high efficiency and long lifetime of thermally activated delayed fluorescent organic light-emitting diodes. J. Mater. Chem. C 2021, 9, 7328–7335.
- Yu, R.; Song, Y.; Chen, M.; He, L. Green to blue-green-emitting cationic iridium complexes with a CF3-substituted phenyl-triazole type cyclometalating ligand: Synthesis, characterization and their use for efficient light-emitting electrochemical cells. Dalton Trans. 2021, 50, 8084–8095.
- Petdee, S.; Chaiwai, C.; Benchaphanthawee, W.; Nalaoh, P.; Kungwan, N.; Namuangruk, S.; Sudyoadsuk, T.; Promarak, V. Imidazole-based solid-state fluorophores with combined ESIPT and AIE features as self-absorption-free non-doped emitters for electroluminescent devices. Dyes Pigments 2021, 193, 109488.
- Luo, J.D.; Xie, Z.L.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.Y.; Qiu, C.F.; Kwok, H.S.; Zhan, X.W.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741.
- Chen, J.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.; Lo, S.M.F.; Williams, I.D.; Zhu, D.; Tang, B.Z. Synthesis, Light Emission, Nanoaggregation, and Restricted Intramolecular Rotation of 1,1-Substituted 2,3,4,5-Tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546.
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353.
- Niu, Y.; Peng, Q.; Deng, C.; Gao, X.; Shuai, Z. Theory of Excited State Decays and Optical Spectra: Application to Polyatomic Molecules. J. Phys. Chem. A 2010, 114, 7817–7831.
- Peng, Q.; Niu, Y.; Deng, C.; Shuai, Z. Vibration correlation function formalism of radiative and non-radiative rates for complex molecules. Chem. Phys. 2010, 370, 215–222.
- Shuai, Z.; Peng, Q. Excited states structure and processes: Understanding organic light-emitting diodes at the molecular level. Phys. Rep. 2014, 537, 123–156.
- Li, K.; Liu, Y.; Feng, Q.; Hou, H.; Tang, B.Z. 2,5-bis(4-alkoxycarbonylphenyl)-1,4-diaryl-1,4-dihydropyrrolo[3,2-b]pyrrole (AAPP) AIEgens: Tunable RIR and TICT characteristics and their multifunctional applications. Chem. Sci. 2017, 8, 7258–7267.
- Han, T.; Yan, D.; Wu, Q.; Song, N.; Zhang, H.; Wang, D. Aggregation-Induced Emission: A Rising Star in Chemistry and Materials Science. Chin. J. Chem. 2021, 39, 677–689.
- Zhang, T.; Zhu, G.; Lin, L.; Mu, J.; Ai, B.; Li, Y.; Zhuo, S. Cyano substitution effect on the emission quantum efficiency in stilbene derivatives: A computational study. Org. Electron. 2019, 68, 264–270.
- Shuai, Z.; Wang, D.; Peng, Q.; Geng, H. Computational Evaluation of Optoelectronic Properties for Organic/Carbon Materials. Acc. Chem. Res. 2014, 47, 3301–3309.
- Zhang, T.; Jiang, Y.; Niu, Y.; Wang, D.; Peng, Q.; Shuai, Z. Aggregation Effects on the Optical Emission of 1,1,2,3,4,5-Hexaphenylsilole (HPS): A QM/MM Study. J. Phys. Chem. A 2014, 118, 9094–9104.
- Li, Q.; Blancafort, L. A conical intersection model to explain aggregation induced emission in diphenyl dibenzofulvene. Chem. Commun. 2013, 49, 5966–5968.
- Crespo-Otero, R.; Li, Q.; Blancafort, L. Exploring Potential Energy Surfaces for Aggregation-Induced Emission—From Solution to Crystal. Chem. Asian J. 2019, 14, 700–714.
- Hu, L.; Farrokhnia, M.; Heimdal, J.; Shleev, S.; Rulíšek, L.; Ryde, U. Reorganization Energy for Internal Electron Transfer in Multicopper Oxidases. J. Phys. Chem. B 2011, 115, 13111–13126.
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907.
- Roy, E.; Nagar, A.; Chaudhary, S.; Pal, S. Advanced Properties and Applications of AIEgens-Inspired Smart Materials. Ind. Eng. Chem. Res. 2020, 59, 10721–10736.
- Tang, B.Z.; Zhan, X.; Yu, G.; Sze Lee, P.P.; Liu, Y.; Zhu, D. Efficient blue emission from siloles. J. Mater. Chem. 2001, 11, 2974–2978.
- Li, J.; Bisoyi, H.K.; Lin, S.; Guo, J.; Li, Q. 1,2-Dithienyldicyanoethene-Based, Visible-Light-Driven, Chiral Fluorescent Molecular Switch: Rewritable Multimodal Photonic Devices. Angew. Chem. Int. Ed. 2019, 58, 16052–16056.
- Puthanveedu, A.; Shanmugasundaram, K.; Yoon, S.; Choe, Y. Thenil and furil-imidazole-based efficient ionic green emitters with high color purity for non-doped light-emitting electrochemical cells. J. Mater. Chem. C 2021.
- Morvillo, P.; Grimaldi, I.A.; Diana, R.; Loffredo, F.; Villani, F. Study of the microstructure of inkjet-printed P3HT:PCBM blend for photovoltaic applications. J. Mater. Sci. 2012, 48, 2920–2927.
- Emami, M.; Shahroosvand, H.; Bikas, R.; Lis, T.; Daneluik, C.; Pilkington, M. Synthesis, Study, and Application of Pd(II) Hydrazone Complexes as the Emissive Components of Single-Layer Light-Emitting Electrochemical Cells. Inorg. Chem. 2021, 60, 982–994.
- Liu, Y.; Xiao, X.; Ran, Y.; Bin, Z.; You, J. Molecular design of thermally activated delayed fluorescent emitters for narrowband orange–red OLEDs boosted by a cyano-functionalization strategy. Chem. Sci. 2021.
- Park, J.M.; Nam, S.H.; Hong, K.-I.; Jeun, Y.E.; Ahn, H.S.; Jang, W.-D. Stimuli-responsive fluorescent dyes for electrochemically tunable multi-color-emitting devices. Sens. Actuators B Chem. 2021, 332, 129534.
- Sayed, M.; Tom, D.M.; Pal, H. Multimode binding and stimuli responsive displacement of acridine orange dye complexed with p-sulfonatocalix[4/6]arene macrocycles. Phys. Chem. Chem. Phys. 2020, 22, 13306–13319.
- Diana, R.; Caruso, U.; Tuzi, A.; Panunzi, B. A Highly Water-Soluble Fluorescent and Colorimetric pH Probe. Crystals 2020, 10, 83.
- Liu, Z.; Jiang, Z.; Xu, C.; Chen, B.; Zhu, G. Fluorenyl-difluoroboron-β-diketonates with multi-stimuli fluorescent response behavior and their applications in a thermochromic logic gate device. Dyes Pigments 2021, 186, 108990.
- Li, Z.; Dong, Y.; Mi, B.; Tang, Y.; Häussler, M.; Tong, H.; Dong, Y.; Lam, J.W.Y.; Ren, Y.; Sung, H.H.Y.; et al. Structural Control of the Photoluminescence of Silole Regioisomers and Their Utility as Sensitive Regiodiscriminating Chemosensors and Efficient Electroluminescent Materials. J. Phys. Chem. B 2005, 109, 10061–10066.
- Yoon, S.-J.; Chung, J.W.; Gierschner, J.; Kim, K.S.; Choi, M.-G.; Kim, D.; Park, S.Y. Multistimuli Two-Color Luminescence Switching via Different Slip-Stacking of Highly Fluorescent Molecular Sheets. J. Am. Chem. Soc. 2010, 132, 13675–13683.
- Zhu, L.; Zhu, B.; Luo, J.; Liu, B. Design and Property Modulation of Metal–Organic Frameworks with Aggregation-Induced Emission. ACS Mater. Lett. 2021, 3, 77–89.
- Jin, J.; Xue, J.; Liu, Y.; Yang, G.; Wang, Y.-Y. Recent progresses in luminescent metal–organic frameworks (LMOFs) as sensors for the detection of anions and cations in aqueous solution. Dalton Trans. 2021, 50, 1950–1972.
- Wang, S.; Zhang, C.-H.; Zhang, P.; Chen, S.; Song, Z.-L.; Chen, J.; Zeng, R. Rational design of a nanoassembly for activatable fluorescence detection of HAase and imaging in tumor cells. Anal. Methods 2021, 13, 2030–2036.
- Deshpande, N.U.; Virmani, M.; Jayakannan, M. An AIE-driven fluorescent polysaccharide polymersome as an enzyme-responsive FRET nanoprobe to study the real-time delivery aspects in live cells. Polym. Chem. 2021, 12, 1549–1561.
- Li, Y.; Zhong, H.; Huang, Y.; Zhao, R. Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging. Molecules 2019, 24, 4593.
- Shi, H.; Liu, J.; Geng, J.; Tang, B.Z.; Liu, B. Specific Detection of Integrin αvβ3 by Light-Up Bioprobe with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 9569–9572.
- Qin, W.; Ding, D.; Liu, J.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible Nanoparticles with Aggregation-Induced Emission Characteristics as Far-Red/Near-Infrared Fluorescent Bioprobes for In Vitro and In Vivo Imaging Applications. Adv. Funct. Mater. 2011, 22, 771–779.
- Guo, B.; Huang, Z.; Shi, Q.; Middha, E.; Xu, S.; Li, L.; Wu, M.; Jiang, J.; Hu, Q.; Fu, Z.; et al. Organic Small Molecule Based Photothermal Agents with Molecular Rotors for Malignant Breast Cancer Therapy. Adv. Funct. Mater. 2019, 30, 1–11.
- Hu, X.; Zhao, X.; He, B.; Zhao, Z.; Zheng, Z.; Zhang, P.; Shi, X.; Kwok, R.T.K.; Lam, J.W.Y.; Qin, A.; et al. A Simple Approach to Bioconjugation at Diverse Levels: Metal-Free Click Reactions of Activated Alkynes with Native Groups of Biotargets without Prefunctionalization. Research 2018, 2018, 1–12.
- Mei, J.; Leung, N.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940.
- Khan, I.M.; Niazi, S.; Khan, M.K.I.; Pasha, I.; Mohsin, A.; Haider, J.; Iqbal, M.W.; Rehman, A.; Yue, L.; Wang, Z. Recent advances and perspectives of aggregation-induced emission as an emerging platform for detection and bioimaging. TrAC Trends Anal. Chem. 2019, 119, 115637.
- Chen, M.; Xie, W.; Li, D.; Zebibula, A.; Wang, Y.; Qian, J.; Qin, A.; Tang, B.Z. Utilizing a Pyrazine-Containing Aggregation-Induced Emission Luminogen as an Efficient Photosensitizer for Imaging-Guided Two-Photon Photodynamic Therapy. Chem.-Eur. J. 2018, 24, 16603–16608.
- Gondia, N.; Sharma, S. Comparative optical studies of naphthalene based Schiff base complexes for colour tunable application. Mater. Chem. Phys. 2019, 224, 314–319.
- Panunzi, B.; Diana, R.; Caruso, U. A Highly Efficient White Luminescent Zinc (II) Based Metallopolymer by RGB Approach. Polymers 2019, 11, 1712.
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. A symmetrical azo-based fluorophore and the derived salen multipurpose framework for emissive layers. Inorg. Chem. Commun. 2019, 104, 186–189.
- Han, X.; Bai, Q.; Yao, L.; Liu, H.; Gao, Y.; Li, J.; Liu, L.; Liu, Y.; Li, X.; Lu, P.; et al. Highly Efficient Solid-State Near-Infrared Emitting Material Based on Triphenylamine and Diphenylfumaronitrile with an EQE of 2.58% in Nondoped Organic Light-Emitting Diode. Adv. Funct. Mater. 2015, 25, 7521–7529.
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Caruso, U.; Piotto, S. Solid-State Highly Efficient DR Mono and Poly-dicyano-phenylenevinylene Fluorophores. Molecules 2018, 23, 1505.
- Zhou, Z.; Li, W.; Hao, X.; Redshaw, C.; Chen, L.; Sun, W.-H. 6-Benzhydryl-4-methyl-2-(1H-benzoimidazol-2-yl)phenol ligands and their zinc complexes: Syntheses, characterization and photoluminescence behavior. Inorg. Chim. Acta 2012, 392, 345–353.
- Lozano, G. The Role of Metal Halide Perovskites in Next-Generation Lighting Devices. J. Phys. Chem. Lett. 2018, 9, 3987–3997.
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82.
- Caruso, U.; Diana, R.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Facile synthesis of new Pd(II) and Cu(II) based metallomesogens from ligands containing thiophene rings. Inorg. Chem. Commun. 2009, 12, 1135–1138.
- Alam, P.; Climent, C.; Alemany, P.; Laskar, I.R. “Aggregation-induced emission” of transition metal compounds: Design, mechanistic insights, and applications. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100317.
- Xie, Y.-Z.; Shan, G.-G.; Li, P.; Zhou, Z.-Y.; Su, Z.-M. A novel class of Zn(II) Schiff base complexes with aggregation-induced emission enhancement (AIEE) properties: Synthesis, characterization and photophysical/electrochemical properties. Dyes Pigments 2013, 96, 467–474.
- Pan, Y.; Wang, J.; Guo, X.; Liu, X.; Tang, X.; Zhang, H. A new three-dimensional zinc-based metal-organic framework as a fluorescent sensor for detection of cadmium ion and nitrobenzene. J. Colloid Interface Sci. 2018, 513, 418–426.
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling building block. Inorg. Chem. Commun. 2019, 104, 145–149.
- Diana, R.; Panunzi, B.; Tuzi, A.; Caruso, U. Two tridentate pyridinyl-hydrazone zinc(II) complexes as fluorophores for blue emitting layers. J. Mol. Struct. 2019, 1197, 672–680.
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Tuzi, A.; Piotto, S.; Caruso, U. Fluorescence pH-dependent sensing of Zn(II) by a tripodal ligand. A comparative X-ray and DFT study. J. Lumin. 2019, 212, 200–206.
- Zhang, G.; Chen, Q.; Zhang, Y.; Kong, L.; Tao, X.; Lu, H.; Tian, Y.; Yang, J. Bulky group functionalized porphyrin and its Zn (II) complex with high emission in aggregation. Inorg. Chem. Commun. 2014, 46, 85–88.
- Landi, G.; Fahrner, W.R.; Concilio, S.; Sessa, L.; Neitzert, H.C. Electrical Hole Transport Properties of an Ambipolar Organic Compound With Zn-Atoms on a Crystalline Silicon Heterostructure. IEEE J. Electron. Devices Soc. 2014, 2, 179–181.
- Suman, G.R.; Pandey, M.; Chakravarthy, A.J. Review on new horizons of aggregation induced emission: From design to development. Mater. Chem. Front. 2021, 5, 1541–1584.
- Panunzi, B.; Concilio, S.; Diana, R.; Shikler, R.; Nabha, S.; Piotto, S.; Sessa, L.; Tuzi, A.; Caruso, U. Photophysical Properties of Luminescent Zinc(II)-Pyridinyloxadiazole Complexes and their Glassy Self-Assembly Networks. Eur. J. Inorg. Chem. 2018, 2018, 2709–2716.
- Brahma, R.; Baruah, J.B. Self-Assemblies of Zinc Complexes for Aggregation-Induced Emission Luminogen Precursors. ACS Omega 2020, 5, 3774–3785.
- Yan, Y.; Yin, G.-Q.; Khalife, S.; He, Z.-H.; Xu, C.; Li, X. Self-assembly of emissive metallocycles with tetraphenylethylene, BODIPY and terpyridine in one system. Supramol. Chem. 2019, 31, 597–605.
- Dong, Y.-W.; Fan, R.-Q.; Wang, X.-M.; Wang, P.; Zhang, H.-J.; Wei, L.-G.; Chen, W.; Yang, Y.-L. (E)-N-(Pyridine-2-ylmethylene)arylamine as an Assembling Ligand for Zn(II)/Cd(II) Complexes: Aryl Substitution and Anion Effects on the Dimensionality and Luminescence Properties of the Supramolecular Metal–Organic Frameworks. Cryst. Growth Des. 2016, 16, 3366–3378.
- Rouhani, F.; Morsali, A.; Retailleau, P. Simple One-Pot Preparation of a Rapid Response AIE Fluorescent Metal–Organic Framework. ACS Appl. Mater. Interfaces 2018, 10, 36259–36266.
- Hamada, Y.; Sano, T.; Fujita, M.; Fujii, T.; Nishio, Y.; Shibata, K. Blue Electroluminescence in Thin Films of Azomethin-Zinc Complexes. Jpn. J. Appl. Phys. 1993, 32, L511–L513.
- Cibian, M.; Shahalizad, A.; Souissi, F.; Castro, J.; Ferreira, J.G.; Chartrand, D.; Nunzi, J.-M.; Hanan, G.S. A Zinc(II) Benzamidinate N -Oxide Complex as an Aggregation-Induced Emission Material: Toward Solution-Processable White Organic Light-Emitting Devices. Eur. J. Inorg. Chem. 2018, 2018, 4322–4330.
- Shahroosvand, H.; Heydari, L.; Bideh, B.N.; Pashaei, B.; Tarighi, S.; Notash, B. Low-Turn-On-Voltage, High-Brightness, and Deep-Red Light-Emitting Electrochemical Cell Based on a New Blend of [Ru(bpy)3]2+ and Zn–Diphenylcarbazone. ACS Omega 2018, 3, 9981–9988.
- Wu, T.; Xie, M.; Huang, J.; Yan, Y. Putting Ink into Polyion Micelles: Full-Color Anticounterfeiting with Water/Organic Solvent Dual Resistance. ACS Appl. Mater. Interfaces 2020, 12, 39578–39585.
