The cyclic nucleotide cAMP (3′,5′-cyclic adenosine monophosphate) is nowadays recognised as an important signalling molecule in plants, involved in many molecular processes, including sensing and response to biotic and abiotic environmental stresses. The validation of a functional cAMP-dependent signalling system in higher plants has spurred a great scientific interest on the polyhedral role of cAMP, as it actively participates in plant adaptation to external stimuli, in addition to the regulation of physiological processes. The complex architecture of cAMP-dependent pathways is far from being fully understood, because the actors of these pathways and their downstream target proteins remain largely unidentified. Recently, a genetic strategy was effectively used to lower cAMP cytosolic levels and hence shed light on the consequences of cAMP deficiency in plant cells. This review aims to provide an integrated overview of the current state of knowledge on cAMP’s role in plant growth and response to environmental stress. Current knowledge of the molecular components and the mechanisms of cAMP signalling events is summarised.
- abiotic stress
- cAMP
- cyclic nucleotides-gated channels
- plant innate immunity
1. Introduction
The role of 3′,5′-cyclic adenosine monophosphate (cAMP) as second messenger in a wide variety of physiologic responses has long been unravelled in animals, bacteria, fungi and algae. By contrast, comprehensive knowledge of cAMP signal transduction in higher plants is still lacking. However, over the last twenty years, several pieces of evidence about cAMP biological functions in plants have been reported. Recent advances in plant biology research, supported by biochemical, genetic and omic studies, have led to the characterisation of cAMP as a polyhedral molecule, critically involved in the signalling pathways of both plant development and environmental stress response.
The recognition of cAMP existence in mammals was the first step towards the identification of its role in living organisms [1]. Thereafter, the molecular structure and conformation of cAMP, which are key factors defining cAMP chemical properties, the specificity of target recognition sites and hence its biological activity, have been determined [2][3]. cAMP responses are extremely complex: different stimuli able to change cAMP levels might lead to different physiological outcomes [4]. The high level of cell compartmentalisation of cAMP signalling pathways is the physiological basis of such numerous and diversified responses to cAMP, as signal response elements are differentially localised and temporally regulated [5]. In animals, several interconnected signalling pathways encompass cAMP and cyclic nucleotides activity in the regulation of cellular events, such as cell proliferation, differentiation, death and migration, as well as complex functions, e.g., memory [6][7].
Even though earlier comparative studies put forward a similar role for cAMP in plants, compared to mammalian organisms, cAMP presence and activity in plants has been a matter of controversy for decades. In fact, in plant cells, cAMP is present in nanomolar concentrations, which are one order of magnitude lower than in mammalian cells. In early studies, cAMP was hardly detectable because cellular levels were below the detection limits of available analytical methods [8][9]. The conclusive proof of cAMP existence and activity in plant extracts could be achieved later, through the advances in high performance liquid chromatography and electrospray mass spectrometry, with a lower detection limit of 25 femtomoles for cyclic nucleotide quantification [10][11].
More recently, studies in plant cells focused on the biosynthetic molecular components of cAMP production and breakdown, which are able to switch on and off the signal encoded by cAMP. The lifecycle of the cAMP molecule includes a source, several regulatory factors with specific cAMP binding domains to transduce the signal and breakdown enzymes to avoid the accumulation of cAMP and terminate the signal [4]. The identification of plant biosynthetic enzymes was not straightforward and took a lot of efforts because of the low homology with previously characterised animal systems were not straightforward. In animals, cAMP is produced in the cytoplasm from adenosine triphosphate by plasma-membrane associated or soluble adenylate cyclases (ACs). Once generated inside the cell, cAMP transduces signals acting through a few cellular effectors, which are responsible for the divergence of cAMP signalling. Changes in intracellular cAMP levels affect cAMP-dependent protein kinases activity (PKA) [12]. Furthermore, cAMP binds to cyclic nucleotide binding proteins, as cyclic nucleotides-gated channels (CNGCs) and hyperpolarisation-activated cyclic nucleotides-modulated channels [13], or to specific transporters and transcription factors in the nucleus. The cAMP levels are regulated, in terms of both lifetime and cell sub-localisation, by cytoplasmic phosphodiesterases (PDEs), which hydrolyse it into AMP, switching off the signal.
After the discovery of the first plant AC in Agapanthus umbellatus [14], it took considerable efforts before other plant ACs were identified and characterised [15][16][17][18]. Similar to plant guanylate cyclases, also indicated as “moonlighting” proteins [19], which are multifunctional enzymes and hold diverse domain structures, plant ACs also harbour multiple catalytically active AC centres, which co-function with other functional domains [18][20][21][22].
Although physiological and biochemical studies provided evidence for enzyme activation by cyclic nucleotides [23], the lack of genetic information on their molecular identity has hitherto prevented the characterisation of PDEs and PKAs orthologs in plants. However, both PDEs and PKAs are postulated to form complexes with other enzymes [9][23]. There is only one molecularly confirmed PDE in liverwort Marchantia polymorpha, which exhibits both AC and PDE activities, but no homologues were found in other plant species [24]. Moreover, many studies point to light PKA activity in many plant species, but these observations still await molecular confirmation [23].
Cyclic nucleotides have a direct effect on cation fluxes (K+, Na+ and Ca2+), and CNGCs are key components of cAMP signal transduction pathways [25]. These ion channels take part to plant reproductive processes, leaf senescence and plant responses to abiotic and biotic stresses [26][27][28][29][30]. They are sensitive to intracellular alterations of the cAMP level and can turn cAMP variations into changes in membrane potential and ion concentrations. CNGCs have different cellular localisation, thus defining the spatial regulation of intracellular cAMP levels.
These findings on plant cAMP biosynthesis and regulation shed light on cAMP role and cAMP-dependent signal transduction mechanisms in plants. However, the understanding of cellular function requires an integrated analysis of context-specific, spatiotemporal data from diverse sources. In this context, the availability of more reliable methods to monitor and/or alter intracellular cAMP levels, without interfering with cell physiological processes, is of utmost importance. Indeed, the roles of cAMP in plants have been mainly established by studies that utilise pharmacological approaches. A recently developed non-invasive method to alter cellular cAMP levels overcame the concern about the effects exerted by the high non-physiological concentrations of exogenously applied cAMPs analogues in both animals and plant systems [31][32][33].
Ion homeostasis [34][35][36], cell division [37][38], pollen tube growth and reorientation [14] and stomatal opening [39][40] are all plant processes involving cAMP level alterations. Proteomic analyses on Arabidopsis plants highlighted the involvement of cAMP in the regulation of photosynthesis and photorespiration, as well as in the energy-transducing pathways and ATP generation [41][42][43]. These studies, while unravelling cAMP role in plant cell development and growth, also pointed out the unavoidable influence of environment in plant life, emphasising cAMP involvement in perception of abiotic and biotic stimuli and in boosting plant stress responses.
2. cAMP Involvement in Plant Response to Abiotic Stress
The establishing of plant responses to environmental stimuli requires the activation of multiple reactions at gene, transcript and protein level, interconnected by the action of signalling messengers [44]. In environmentally stressed plants, cellular metabolism faces a remarkable rearrangement allowing stress acclimation. Early alarm stages of plant abiotic stress response include the onset of oxidative stress and the induction of stress-responsive signalling pathways. Following the acclimation phase, with the biosynthesis of stress-protective compounds, cells encounter new recovering homeostasis, at the expense of cellular energy [44][45][46]. In this scenario, cAMP may act as stress sensors and/or modulator of cellular metabolism, mainly, but not only, through its influence on ion channels and the resulting regulation of ion fluxes [16] (Table 1).
Table 1. Proposed role of cAMP in the acquisition of stress tolerance.
| Stress | Mechanisms | Molecular Players | References |
|---|---|---|---|
| Salinity | Limitation of Na+ influx | VICs; CNGCs | [47][48] |
| Aluminium | K+ current permitting malate outflux | Cation channels | [49] |
| K+ deficiency | K+ homeostasis regulation | AtKUP5; AtKUP7; CNGCs. | [20][21] |
| Heat | Ca2+ influx and HSPs expression | CNGCs; HSPs. | [50] |
| Drought | Synthesis of protective polypeptides | ABA signalling | [51] |
| Wounding | Regulations of the phenylpropanoid pathway | PAL; 4CL; CHS. | [52] |
| ROS | Reduction of Ca2+ influx and K+ efflux | CNGCs | [48] |
Abbreviations: ABA, abscisic acid; CHS, chalcone synthase; 4CL, 4-coumarate:coenzyme A ligase; CNGCs, cyclic nucleotide gated channels; HSPs, heat shock proteins; PAL, phenylalanine ammonia lyase; VICs, voltage-independent non-selective channels.
In Arabidopsis, the improvement of plant salinity tolerance involves cAMP, which causes the deactivation of voltage-independent non-selective channels, limiting Na+ influx [47]. In wheat, tolerance to aluminium requires cAMP-dependent outward-rectifying K+ current, which permits malate outflow that chelates this toxic metal [49].
The important link between K+ flux and cAMP production was further defined in Arabidopsis thaliana by the isolation and characterisation of two K+-uptake permeases, AtKUP5 and AtKUP7. Both the K+-uptake permeases have a dual function, harbouring also a functional AC catalytic domain [20]. AtKUP7 is a K+ transporter in roots, functionally active under K+-limited conditions [53][54]. In addition, AtKUP7 was defined as a proton-coupled carrier with AC function, but it is still unclear if cAMP production is dependent on K+ fluxes and/or if cAMP can modulate K+ fluxes [20]. AtKUP5 causes a K+ flux-dependent cAMP accumulation in the cytosol, which can in turn activate downstream components essential for K+ homeostasis, including CNGCs [21].
cAMP involvement in abiotic stress response often goes through the regulation of CNGCs [29]. Remarkably, these ion channels, having overlapped binding domains for cyclic nucleotides and calmodulin, favour the crosstalk between the signalling of these second messengers [55][56]. Functional characterisation of Arabidopsis CNGC2 shows that cAMP activation of AtCNGC2 currents could be reversed by calmodulin, suggesting that the physical interaction of Ca2+ and calmodulin with CNGCs stops cyclic nucleotide activation of the channels. Therefore, the cytosolic cAMP, Ca2+ and calmodulin can operate in an integrated way to gate currents through CNGCs. [57].
CNGCs allow the influx of K+, Na+ and Ca2+ into the cell, with different selectivities; hence, they work downstream the environmental stimuli perception to mediate plant tolerance to drought, salinity and extreme temperature, which affect ionic and osmotic cellular homeostasis [29]. AtCNGC2 was shown to partially complement the yeast mutant at low K+ concentration only in the presence of membrane-permeable cAMP [58]. AtCNGC10, AtCNGC19 and AtCNGC20 were shown to be involved in plant tolerance to salt stress [59][60]. The antisense lines of AtCNGC10 showed altered K+ and Na+ levels in shoots and were less tolerant to salt stress [59]. AtCNGC19 and AtCNGC20, participating in the re-allocation of Na+ in the plants, might permit their survival to high salt levels [60].
Arabidopsis CNGC16 was shown to confer thermotolerance to germinating pollen, linking cyclic nucleotide signalling to heat stress response. In the cngc16 mutants, the reduced transmission of pollen at high temperature was linked to a weakened expression of crucial stress-responsive genes. [61]. The role of CNGCs in plant thermotolerance was also validated in the vegetative tissue of plants. Mutants in CNGC2 showed hypersensitive heat-responsive Ca2+ influx, which conferred acquired thermotolerance at milder heat stress than in wild-type plants [62]. Mutation in Arabidopsis CNGC6 led to impaired heat stress response, which suggests its involvement in the acquisition of thermotolerance [50]. In addition, in Arabidopsis, it was shown that a heat shock caused an increase in intracellular cAMP levels, which, in turn, stimulating CNGC6, triggered a cytosolic Ca2+ influx. Furthermore, the treatment with an exogenous cAMP analogue induced the expression of some heat shock proteins, indicating the contribution of this second messenger in plant heat stress response [50].
Proteomic studies also supported a role of cAMP in controlling plant response to temperature, as well as to light. Thomas, Alqurashi, and their colleagues, suggested that cAMP participates as signalling molecule to the photosynthetic process of acclimation. [41][42]. The analyses revealed that, after cAMP treatment, the most enriched proteins belonged to the GO categories “Response to stress”, “Response to abiotic stimulus”, “Response to salt” and “Response to cold”. Moreover, there was an enrichment of the category “Photosynthesis and light reaction processes” in both up- and downregulated cAMP responsive genes [41]. cAMP involvement in photosynthetic pathways was also described by Donaldoson and colleagues [43], who reported the interaction between cAMP and enzymes involved in Calvin cycle and photorespiration pathway. This is of interest since in Nicotiana tabacum, through a quantitative method based on mass spectrometric analysis, AC activity was observed in chloroplasts [63]. Moreover, in oat seedlings, it was shown that light influenced cAMP accumulation, pointing out that cAMP could take part in the phytochrome signalling pathway [64].
A role for cAMP in plant response to drought was also proposed in wheat. Indeed, the exogenous application of both cAMP and ABA promoted the synthesis of polypeptides whose accumulation is stimulated by dehydration, suggesting that cAMP signalling is possibly involved in the effect of ABA on protein synthesis during drought [51].
cAMP was shown to be involved in response to wounding in Hippeastrum x hybridum. In this plant, the transcriptional activity of the HpAC1 gene, which encodes a functional AC, as well as the level of cAMP, showed two peaks in response to mechanical damage. The authors proposed that the first rapid induction of HpAC1, and the concomitant transient changes in cAMP, might function as an “alarm” that alerts plant cells against the damage. The later increase in HpAC1 expression and cAMP accumulation might be linked to the induction of systemic responses and, in particular, to the induction of phenylalanine ammonia lyase (PAL) involved in the production of phytoalexins, which protect damaged tissue against potential pathogen attacks [52]. Together with PAL induction, cAMP was shown to be involved in the stimulation of the expression of 4-coumarate:coenzyme A ligase and chalcone synthase, enzymes of the phenylpropanoid pathway, which participates to plant response to a multiplicity of environmental stimuli, including nutrient depletion, UV irradiation, extreme temperatures and heavy metal toxicity [65].
Oxidative stress is a common feature associated with various abiotic stress factors, and reactive oxygen species (ROS) have an important biological role in sensing and activating acclimation mechanisms [44][66][67]. The superoxide-generating NADPH oxidase integrates Ca2+ and ROS signalling, which in turn may be connected to cyclic nucleotides through CNGCs [68]. Each messenger mutually enhances the induction of the other during abiotic stress conditions, resulting in the propagation of ROS and Ca2+ waves across the plasma membrane to establish the proper acclimation response, to which cAMP may directly or indirectly participate [69][70].
A correlation among cAMP, ROS and ion homeostasis was demonstrated in plant response to salt stress [48]. Several studies indicated that, in roots under salt stress, ROS accumulation could be due to the disturbance of mitochondrial function, as well as to activation of NADPH oxidases [71][72]. Furthermore, under salt stress, Na+-influx into the cell causes a significant loss of cytosolic K+, which can be responsible for important metabolic alterations [73][74]. The treatment of Arabidopsis roots with H2O2 induced a rapid Ca2+-influx and K+-efflux, which were reduced by pre-treatment with cAMP. Moreover, coherently with the accumulation of H2O2 level in salt-stressed roots [71][72], pre-treatment with cAMP decreased salt-dependent K+-efflux [70]. Ordonez and colleagues proposed that CNGCs, proved to be involved in plant responses to salt stress [60], could be in part responsible of the H2O2-dependent K+- efflux, which was reduced by cyclic nucleotides [48].
3. Role of cAMP in Plant Innate Immunity
Plants are continuously exposed to a variety of invading microorganisms, including viruses, bacteria and fungi. Although plants are lacking mobile sentinel cells, distinctive of the animal immune systems, they can perceive and keep away pathogens, through a two-layer innate immune system [75]. In the first layer of defence, called pattern-triggered immunity (PTI), membrane pattern recognition receptors (PRRs) recognise pathogen/microbe-associated molecular patterns (PAMPs/MAMPs) or endogenous damage-associated molecular patterns (DAMPs) [76][77]. This recognition initiates a series of defence responses, including ROS production, Ca2+ influx and activation of kinases as Ca2+-dependent protein kinases and mitogen-activated protein kinase, leading to the upregulation of defence genes [77][78]. However, pathogens can secrete into plant cells effectors, namely virulence factors encoded by avirulence (avr) genes, which can suppress PTI. The effector recognition by intracellular receptors encoded by resistance genes activates the second layer of defence, the effector-triggered immunity (ETI). Defence responses of ETI are typically stronger than PTI and often culminate with the hypersensitive response (HR), a form of programmed cell death, occurring at the infection site with the aim to narrow pathogen infection [75][79]. An increase in the antimicrobial phytoalexins, as well as in salicylic acid (SA) and pathogenesis-related (PR) proteins, occurs locally in the site of infection, and systemically in uninfected tissues [80].
Several studies indicated the involvement of cAMP in plant immune response [33][81][82][83][84][85][86]. Considering all the literature data until now reported, possible cAMP-mediated mechanisms activated during plant-immunity are discussed (Figure 1).
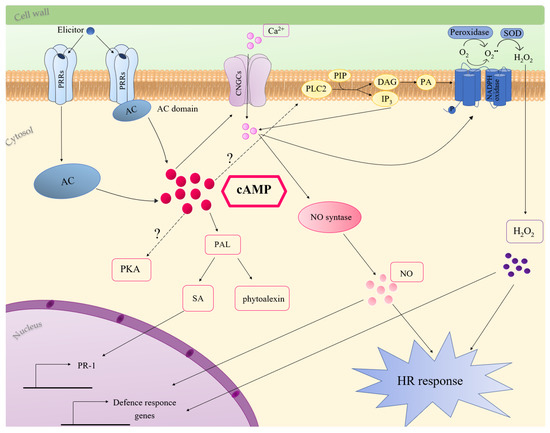
Figure 1. Molecular mechanisms of cAMP involvement in plant innate immunity. Elicitor recognition elevates cytosolic cAMP, which can activate CNGCs or PLC2, inducing Ca2+ accumulation and oxidative burst, through the activation of NADPH oxidase. cAMP-dependent oxidative burst can also be due to apoplastic peroxidases. Ca2+ stimulates NO production, which, together with ROS, induces defence response and HR. cAMP accumulation also activates PAL expression and production of SA and phytoalexins. More details are provided in the text. Question marks indicate pathways not completely characterised. Abbreviations: AC, adenylate cyclase; cAMP, 3′,5′-cyclic adenosine monophosphate; CNGCs, cyclic nucleotides-gated channels; DAG, diacylglycerol; HR, hypersensitive response; IP3, inositol triphosphate; NO, nitric oxide; PA, phosphatidic acid; PAL, phenylalanine ammonia lyase; PIP, monophosphatidylinosotol; PKA, protein kinase A, PLC2, phospholipase C2; PR-1, pathogenesis-related genes; PRRs, pattern recognition receptors; SA, salicylic acid; SOD, superoxide dismutase.
Initially, a role for cAMP in the biosynthesis of phytoalexins was proposed. In carrot cell culture, the addition of the permeable dibutyryl cAMP, or forskolin and cholera toxin, activators of adenylate cyclase and G proteins, respectively, induced the biosynthesis of the antifungal phytoalexin 6-methoxymellein. Interestingly, the cAMP-dependent production of this phytoalexin was inhibited by Ca2+ channel blockers, as well as by inhibitors of calmodulin-dependent processes, suggesting that the increase in cAMP content in carrot cells induces Ca2+ influx across the plasma membrane [87][81]. In Cupressus lusitanica cell cultures, cAMP is involved in elicitor-induced production of the phytoalexin, β-thujaplicin. The authors suggested that cAMP-dependent β-thujaplicin accumulation involves Ca2+ and K+ fluxes since it was inhibited by K+ and Ca2+ channel blockers. This study also indicated a contribution of protein kinase cascades in cAMP signalling processes leading to β-thujaplicin accumulation [83]. The cAMP-dependent production of phytoalexins was also shown in Medicago sativa. In this case, the treatment with an elicitor of the phytopathogenic fungus, Verticillium alboatrum, caused a dose-dependent increase in the activity of AC and in intracellular cAMP content. Moreover, the treatment of Medicago cells with cAMP enhanced PAL activity and the synthesis of the phytoalexin medicarpin [84]. Consistently, in Arabidopsis, the treatment of seedlings with the permeable cAMP analogue 8-Br-cAMP increased, up to 40-fold and 2-fold, respectively, the expression of PAL2 and PAL1 [63]. PAL, the expression of which increased in response to diverse pathogens and elicitors, also plays a key role in SA synthesis [88][89][90] (Figure 1). Remarkably, cAMP elevation in Arabidopsis increased the endogenous SA level in response against Verticillium secreted toxins. The treatment of Arabidopsis with an AC inhibitor strongly reduced SA accumulation and PR-1 expression caused by Verticillium toxins. Both 8-Br-cAMP and SA enhanced resistance of Arabidopsis to the toxins, but cAMP acts upstream SA, since it was not able to potentiate the resistance of Arabidopsis plants deficient in SA [84]. In line with a role for cAMP in SA-dependent defence responses, the upregulation of PR-1 gene expression, occurring in response to an avirulent strain of Pseudomonas syringae, was decreased in cAS plants with low cAMP levels [33].
During plant immune responses, an oxidative burst arises in two phases, the first occurring within few minutes after pathogen perception and the second occurring later and with a higher amplitude [91]. ROS play several roles in response to pathogens, such as the reinforcement of cell wall, the activation MAP kinase pathways, the induction of HR and the triggering of systemic responses [92][93]. Two main mechanisms including NADPH oxidases and peroxidases have been proposed for ROS generation in response to pathogens [93][94]. Many literature data suggest an involvement of cAMP in pathogen/elicitor induced oxidative burst (Figure 1). In French bean cell culture, cAMP level increased upon the addition of an elicitor of the fungus Colletotrichum lindemuthianum and cAMP itself induced ROS accumulation. The cAMP-mediated apoplastic oxidative burst was increased by cholera toxin and inhibited by Ca2+ channel blockers. Bindschedler and co-workers suggested that G proteins and cAMP are involved in extracellular alkalisation and Ca2+ influx, essential for the pH-dependent apoplastic peroxidases, which mediate the oxidative burst [95]. Likewise, the treatment of Arabidopsis thaliana cells with forskolin enhanced the oxidative burst occurring in response to an elicitor from Fusarium oxysporum [96]. ROS generation induced by the PAMP lipopolysaccharide in Arabidopsis was prevented by the addition of an AC inhibitor [96]. Similarly, cAMP dampening in Arabidopsis cAS plants caused a delay in H2O2 increase at the early stage of response to an avirulent strain of Pseudomonas syringae [33].
Genetic evidence supports a role for CNGCs in pathogen-induced HR and disease resistance (Figure 3). In Arabidopsis, the mutation in DND1 (defence-no-death), which encodes AtCNGC2, failed to induce HR in response to an avirulent strain of P. syringae. Moreover, dnd1 mutants showed constitutive systemic resistance and elevated levels of SA [97]. HLM1, encoding AtCNGC4, which works as a K+- and Na+-permeable channel activated by cGMP or cAMP, was upregulated in response to pathogen infection. hlm1 mutant plants showed a lesion-mimic phenotype and an altered HR in response to avirulent P. syringae pv tomato (Pst) strains harbouring the avrRps4 or avrRpm1 genes [98]. In Arabidopsis, cAMP-activated AtCNGC11 and AtCNGC12 are positive mediators of resistance against the avirulent Hyaloperonospora parasitica. In the cpr22 (constitutive expresser of PR genes22) mutant, a 3-kb deletion that fuses AtCNGC11 and AtCNGC12, generates the chimeric gene ATCNGC11/12, which confers the constitutive activation of defence responses [99].
An increase in cytosolic Ca2+, due to influx across the plasma membrane or to efflux from intracellular stores, represents a primary event in plant immune signalling [100][101][102][103]. Interestingly, in dnd1 mutant cells, the deficiency of cAMP-activated inward Ca2+ influx is associated with reduced production of nitric oxide (NO) [104], which was defined as the concertmaster in the HR and defence-gene activation [105][106]. dnd1 mutants showed a weakened HR, and the addition of exogenous NO complements this phenotype [104]. Application of pathogens or PAMPS elevated cytosolic cAMP and the addition of exogenous cAMP led to Ca2+ elevation, NO generation and defence response in the absence of the non-self pathogen signal. Inoculation of dnd1 plants with Pst containing the avrRpm1 or avrRpt2 genes led to a reduction in Ca2+ influx and to an impairment in immune response [104][85]. The weakening of pathogen-associated cytosolic Ca2+ influx also occurred by blocking cAMP synthesis in plants exposed to the pathogen, with a corresponding impairment in HR. On the contrary, co-infiltration with IBMX along with avirulent pathogens enhanced plant immune response, increasing HR. Thus, it was suggested that elevation of cytosolic cAMP, acting upstream from Ca2+, is a key signal in the transduction of pathogen perception and in the downstream signalling cascade of defence responses [85]. Furthermore, the cAMP dampening, occurring in Arabidopsis cAS plants, delayed cytosolic Ca2+ elevation and reduced HR in response to PstAvrB. Sabetta and co-workers suggested that the delay in Ca2+ elevation could be due to a failure in the activation of CNGCs, but also to the down-accumulation of phospholipase C2 (PLC2) occurring in cAS plants [33] (Figure 3). Consistently, it is known that cytosolic Ca2+ accumulation in response to numerous elicitors of plant defence involves phosphatidylinositol-specific PLCs [103]. Moreover, since it was reported that PLCs significantly contribute to pathogen/elicitor induced oxidative burst [107][108][109], the low level of PLC2 in cAS plants could also contribute to the delayed H2O2 increase in the first phase of PstAvrB infection [33]. The low availability of cAMP, and the subsequent delay in Ca2+ influx, could be responsible for an incorrect temporal modulation of the AtSR1 [33], a Ca2+-dependent calmodulin binding transcription factor, repressing the expression of target genes [109][110][111]. Consequently, some defence proteins, such as HSP90, CRK14 and DJ1E [112][113][114][115], were not accumulated in cAS cells after pathogen infection, weakening defence response [33].
The involvement of cAMP in plant immunity was supported by the isolation of ACs involved in plant response to pathogens. The silencing of NbAC, a gene encoding an AC in Nicotiana benthamiana, suppresses the necrotic lesions induced by tabtoxinine-β-lactam, a non-specific bacterial toxin, produced by P. syringae pv. Tabaci [116]. The expression of HpAC1, a gene encoding an AC from Hippeastrum x hybridum, and the levels of cAMP, increased in response to Phoma narcissi infection [52]. Recently, a leucine-rich repeat protein, AtLRRAC1, harbouring multiple catalytically active AC centres, was identified in Arabidopsis. AtLRRAC1 was able to complement AC-deficient Escherichia coli and to generate cAMP in vitro [18][22]. Interestingly, atlrrac1 mutants showed compromised immune responses to biotrophic fungi and hemibiotrophic bacteria. The expression of early-induced immune-related genes after elicitation with the PAMP flg22 was strongly inhibited in atlrrac1 plants, suggesting an involvement of AtLRRAC1 in PTI [22].
4. Conclusions
cAMP is the object of intense scientific interest, both in animal systems, where much more progress was achieved in defining its role, and in plants, becoming lately the centre of a bustling research. cAMP is nowadays recognised as a relevant signalling molecule in plant development as well as in responses to environmental stimuli, of both biotic and abiotic nature. As cAMP-signalling networks and their spatial and temporal regulation are extremely complex, future research must deal with the nature of cAMP signals in terms of strength, duration and frequency, considering also the crosstalk between this second messenger and other intracellular regulators [117]. Since the existence of cAMP-regulated processes in plants and the first evidence of compartmentalised cAMP signals in animals, the need for reliable cAMP detection methods able to reveal cAMP waves in living systems arose. Recent advances in modern biotechnologies and synthetic biology, alongside newly developed detection methods and instrumentations, offer a wide range of possibilities to unravel cAMP role in living cells.
The cAMP-sponge represents a cutting-edge genetically encoded tool, used to exploit cAMP fluctuations for the first time in living plant organisms and specific cell compartments. It overcomes major concerns on biochemical assays and pharmacological studies performed so far in plants [31][32][33]. Other developed genetically-encoded tools employed in bacteria and isolated plant cells are the promoter reporter systems, based on the plant protein Oligopeptide TransporterX promoter, which measure alterations in downstream gene expression following changes in intracellular levels of cyclic nucleotides. Unfortunately, this system cannot discriminate between cGMP and cAMP [118].
Taking advantage of the progress reached in animal systems, many other strategies and their combination may help in elucidating cAMP signalling in plant systems. Indeed, optogenetic approaches and genetically encoded fluorescent biosensors are effectively used to monitor and modulate cAMP levels [119][120]. Photoactivated ACs and light-regulated PDEs, or even their association, are successfully used in animal cells [121][122]. The generation of stable plant lines, expressing the combination of optimised sensors for cAMP and concomitant or downstream messengers, may provide a comprehensive view of the signalling event investigated.
Another important requirement is a clear identification and functional characterisation of cAMP-binding proteins involved in the signalling of this second messenger. Nowadays, many lines of evidence indicate that, in plants, the conversion of cAMP into Ca2+ signals via CNGCs is the main signalling mechanism of this cyclic nucleotide. However, although indications for bona fide PKA are lacking, its presence in plants cannot be excluded. New bioinformatics algorithms and molecular tools may provide opportunities to extend the presently scarce knowledge of cAMP-dependent protein kinases [16][23]. Moreover, studies on cAMP-dependent changes in transcriptomes, proteomes and phosphoproteomes, as well as metabolomes, will improve the understanding of cAMP involvement in plant physiological processes, along with acclimation to adverse environmental conditions.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21144862
References
- Rall, T.W.; Sutherland, E.W.; Berthet, J. The relation of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J. Biol. Chem. 1957, 224, 1987–1995.
- Shabb, J.B.; Corbin, J.D. Cyclic nucleotide-binding domains in proteins having diverse functions. J. Biol. Chem. 1992, 267, 5723–57236.
- Newton, R.P.; Smith, C.J. Cyclic nucleotides. Phytochemistry 2004, 65, 2423–2437, doi:10.1016/j.phytochem.2004.07.026.
- Gancedo, J.M. Biological roles of cAMP: Variations on a theme in the different kingdoms of life. Biol. Rev. Camb. Philos. Soc. 2013, 88, 645–668, doi:10.1111/brv.12020.
- Arora, K.; Sinha, C.; Zhang, W.; Ren, A.; Moon, C.S.; Yarlagadda, S.; Naren, A.P. Compartmentalization of cyclic nucleotide signaling: A question of when, where, and why? Pflugers Arch. 2013, 465, 1397–1407, doi:10.1007/s00424-013-1280-6.
- Cheepala, S.; Hulot, J.S.; Morgan, J.A.; Sassi, Y.; Zhang, W.; Naren, A.P.; Schuetz, J.D. Cyclic nucleotide compartmentalization: Contributions of phosphodiesterases and ATP-binding cassette transporters. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 231–235, doi:10.1146/annurev-pharmtox-010611-134609.
- Stork, P.J.S.; Schmitt, J.M. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002, 12, 258–266, doi:10.1016/S0962-8924(02)02294-8.
- Amrhein, N. The current status of cyclic AMP in higher plants. Ann. Rev. Plant Physiol. 1977, 28, 123–132, doi:10.1146/annurev.pp.28.060177.001011.
- Brown, E.G., Newton, R.P. Cyclic AMP and higher plants. Phytochemistry 1981, 20, 2453–2463, doi:10.1016/0031-9422(81)83071-3.
- Witters, E.; Roef, L.; Newton, R.P.; VanDongen, W.; Esmans, E.L.; VanOnckelen, H.A. Quantitation of cyclic nucleotides in biological samples by negative electrospray tandem mass spectrometry coupled to ion suppression liquid chromatography. Rapid Commun. Mass Spectrom. 1996, 10, 225–231, doi:10.1002/(Sici)1097-0231(19960131)10:2<225::Aid-Rcm469>3.3.Co;2-A.
- Witters, E.; Vanhoutte, K.; Dewitte, W.; Machackova, I.; Benkova, E.; Van Dongen, W.; Esmans, E.L.; Van Onckelen, H.A. Analysis of cyclic nucleotides and cytokinins in minute plant samples using phase-system switching capillary electrospray-liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 1999, 10, 143–151, doi:10.1002/(Sici)1099-1565(199905/06)10:3<143::Aid-Pca441>3.3.Co;2-7.
- Beavo, J.A.; Brunton, L.L. Cyclic nucleotide research—Still expanding after half a century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–718, doi:10.1038/nrm911.
- Craven, K.B.; Zagotta, W.N. CNG and HCN channels: Two peas, one pod. Annu. Rev. Physiol. 2006, 68, 375–401, doi:10.1146/annurev.physiol.68.040104.134728.
- Moutinho, A.; Hussey, P.J.; Trewavas, A.J.; Malho, R. cAMP acts as a second messenger in pollen tube growth and reorientation. Proc. Natl. Acad. Sci. USA 2001, 98, 10481–10486, doi:10.1073/pnas.171104598.
- Gehring, C. Adenyl cyclases and cAMP in plant signaling—Past and present. Cell Commun. Signal. 2010, 8, 15, doi:10.1186/1478-811x-8-15.
- Gehring, C.; Turek, I.S. Cyclic Nucleotide monophosphates and their cyclases in plant signaling. Front. Plant Sci. 2017, 8, 1704. 10.3389/Fpls.2017.01704.
- Wong, A.; Tian, X.C.; Gehring, C.; Marondedze, C. Discovery of novel functional centers with rationally designed amino acid motifs. Comput. Struct. Biotechnol. J. 2018, 16, 70–76, doi:10.1016/j.csbj.2018.02.007.
- Ruzvidzo, O.; Gehring, C.; Wong, A. New perspectives on plant adenylyl cyclases. Front. Mol. Biosci. 2019, 6, 136, doi:10.3389/Fmolb.2019.00136.
- Irving, H.R.; Cahill, D.M.; Gehring, C. Moonlighting proteins and their role in the control of signaling microenvironments, as exemplified by cGMP and Phytosulfokine Receptor 1 (PSKR1). Front. Plant Sci. 2018, 9, 415, doi:10.3389/Fpls.2018.00415.
- Al-Younis, I.; Wong, A.; Gehring, C. The Arabidopsis thaliana K+-uptake permease 7 (AtKUP7) contains a functional cytosolic adenylate cyclase catalytic centre. FEBS Lett. 2015, 589, 3848–3852, doi:10.1016/j.febslet.2015.11.038.
- Al-Younis, I.; Wong, A.; Lemtiri-Chlieh, F.; Schrnockel, S.; Tester, M.; Gehring, C.; Donaldson, L. The Arabidopsis thaliana K+-uptake permease 5 (AtKUP5) contains a functional cytosolic adenylate cyclase essential for K+ transport. Front. Plant Sci. 2018, 9, 1645, doi:10.3389/Fpls.2018.01645.
- Bianchet, C.; Wong, A.; Quaglia, M.; Alqurashi, M.; Gehring, C.; Ntoukakis, V.; Pasqualini, S. An Arabidopsis thaliana leucine-rich repeat protein harbors an adenylyl cyclase catalytic center and affects responses to pathogens. J. Plant Physiol. 2019, 232, 12–22, doi:10.1016/j.jplph.2018.10.025.
- Swiezawska, B.; Duszyn, M.; Jaworski, K.; Szmidt-Jaworska, A. Downstream targets of cyclic nucleotides in plants. Front. Plant Sci. 2018, 9, 1428, doi:10.3389/Fpls.2018.01428.
- Kasahara, M.; Suetsugu, N.; Urano, Y.; Yamamoto, C.; Ohmori, M.; Takada, Y.; Okuda, S.; Nishiyama, T.; Sakayama, H.; Kohchi, T.; et al. An adenylyl cyclase with a phosphodiesterase domain in basal plants with a motile sperm system. Sci. Rep. 2016, 6, 39232, doi:10.1038/Srep39232.
- Ma, Y.; He, K.; Berkowitz, G.A., Editorial: From structure to signalsomes: New perspectives about membrane receptors and channels. Front. Plant Sci. 2019, 10, 682, doi:10.3389/Fpls.2019.00682.
- Martinez-Atienza, J.; Van Ingelgem, C.; Roef, L.; Maathuis, F.J. Plant cyclic nucleotide signalling: Facts and fiction. Plant Signal Behav. 2007, 2, 540–543, doi:10.4161/psb.2.6.4789.
- Ma, W.; Smigel, A.; Walker, R.K.; Moeder, W.; Yoshioka, K.; Berkowitz, G.A. Leaf senescence signaling: The Ca2+-conducting Arabidopsis cyclic nucleotide gated channel2 acts through nitric oxide to repress senescence programming. Plant Physiol. 2010, 154, 733–43, doi:10.1104/pp.110.161356.
- Ma, W.; Berkowitz, G.A. Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytol. 2011, 190, 566–572, doi:10.1111/j.1469-8137.2010.03577.x.
- Jha, S.K.; Sharma, M.; Pandey, G.K. Role of Cyclic nucleotide gated channels in stress management in plants. Curr. Genomics 2016, 17, 315–329, doi:10.2174/1389202917666160331202125.
- Duszyn, M.; Swiezawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Cyclic nucleotide gated channels (CNGCs) in plant signalling-Current knowledge and perspectives. J. Plant Physiol. 2019, 241, 153035, doi:10.1016/J.Jplph.2019.153035.
- Lefkimmiatis, K.; Moyer, M.P.; Curci, S.; Hofer, A.M. “cAMP Sponge”: A buffer for cyclic adenosine 3 ‘, 5 ‘-monophosphate. PLoS ONE 2009, 4, e7649, doi:10.1371/journal.pone.0007649.
- Sabetta, W.; Vannini, C.; Sgobba, A.; Marsoni, M.; Paradiso, A.; Ortolani, F.; Bracale, M.; Viggiano, L.; Blanco, E.; de Pinto, M.C., Cyclic AMP deficiency negatively affects cell growth and enhances stress-related responses in tobacco Bright Yellow-2 cells. Plant Mol. Biol. 2016, 90, 467–483, doi:10.1007/s11103-016-0431-5.
- Sabetta, W.; Vandelle, E.; Locato, V.; Costa, A.; Cimini, S.; Bittencourt Moura, A.; Luoni, L.; Graf, A.; Viggiano, L.; De Gara, L.; et al.. Genetic buffering of cyclic AMP in Arabidopsis thaliana compromises the plant immune response triggered by an avirulent strain of Pseudomonas syringae pv. tomato. Plant J. 2019, 98, 590–606, doi:10.1111/tpj.14275.
- Anderson, J.A.; Huprikar, S.S.; Kochian, L.V.; Lucas, W.J.; Gaber, R.F., Functional expression of a probable Arabidopsis-thaliana potassium channel in Saccharomyces-cerevisiae. Proc. Natl. Acad. Sci. USA 1992, 89, 3736–3740, doi:10.1073/pnas.89.9.3736.
- Bolwell, G.P. Cyclic-AMP, the reluctant messenger in plants. Trends Biochem. Sci. 1995, 20, 492–495, doi:10.1016/S0968-0004(00)89114-8.
- Trewavas, A.J. Plant cyclic AMP comes in from the cold. Nature 1997, 390, 657–658, doi:10.1038/37720.
- Ehsan, H.; Reichheld, J.P.; Roef, L.; Witters, E.; Lardon, F.; Van Bockstaele, D.; Van Montagu, M.; Inze, D.; Van Onckelen, H. Effect of indomethacin on cell cycle dependent cyclic AMP fluxes in tobacco BY-2 cells. FEBS Lett. 1998, 422, 165–169, doi:10.1016/S0014-5793(97)01610-4.
- Ehsan, H.; Roef, L.; Witters, E.; Reichheld, J.P.; Van Bockstaele, D.; Inze, D.; Van Onckelen, H. Indomethacin-induced G1/S phase arrest of the plant cell cycle. FEBS Lett. 1999, 458, 349–353, doi:10.1016/S0014-5793(99)01152-7.
- Curvetto, N. Effect of two cAMP analogs on stomatal opening in Vicia faba: Possible relationship with cytosolic calcium concentration. Plant Physiol. Biochem. 1994, 32, 365–372.
- Jin, X.C.; Wu, W.H. Involvement of cyclic AMP in ABA- and Ca2+-mediated signal transduction of stomatal regulation in Vicia faba. Plant Cell Physiol. 1999, 40, 1127–1133, doi:10.1093/oxfordjournals.pcp.a029497.
- Thomas, L.; Marondedze, C.; Ederli, L.; Pasqualini, S.; Gehring, C. Proteomic signatures implicate cAMP in light and temperature responses in Arabidopsis thaliana. J. Proteom. 2013, 83, 47–59, doi:10.1016/j.jprot.2013.02.032.
- Alqurashi, M.; Gehring, C.; Marondedze, C. Changes in the Arabidopsis thaliana proteome implicate cAMP in biotic and abiotic stress responses and changes in energy metabolism. Int. J. Mol. Sci. 2016, 17, 852, doi:10.3390/Ijms17060852.
- Donaldson, L.; Meier, S.; Gehring, C. The arabidopsis cyclic nucleotide interactome. Cell Commun. Signal. 2016, 14, 10, doi:10.1186/s12964-016-0133-2.
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771, doi:10.3389/fpls.2018.01771.
- Kosova, K.; Vitamvas, P.; Prasil, I.T.; Renaut, J. Plant proteome changes under abiotic stress-contribution of proteomics studies to understanding plant stress response. J. Proteomics 2011, 74, 1301–1322, doi:10.1016/j.jprot.2011.02.006.
- Lamers, J.; van der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635, doi:10.1104/pp.19.01464.
- Maathuis, F.J.; Sanders, D. Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol. 2001, 127, 1617–1625, doi:10.1104/pp.010502.
- Ordonez, N.M.; Marondedze, C.; Thomas, L.; Pasqualini, S.; Shabala, L.; Shabala, S.; Gehring, C. Cyclic mononucleotides modulate potassium and calcium flux responses to H2O2 in Arabidopsis roots. FEBS Lett. 2014, 588, 1008–1015, doi:10.1016/j.febslet.2014.01.062.
- Zhang, W.H.; Ryan, P.R.; Tyerman, S.D. Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiol. 2001, 125, 1459–1472, doi:10.1104/pp.125.3.1459.
- Gao, F.; Han, X.W.; Wu, J.H.; Zheng, S.Z.; Shang, Z.L.; Sun, D.Y.; Zhou, R.G.; Li, B. A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—is involved in heat shock responses. Plant J. 2012, 70, 1056–1069, doi:10.1111/j.1365-313X.2012.04969.x.
- Maksyutova, N.N.; Viktorova, L.V. A comparative study of the effect of abscisic acid and cAMP on protein synthesis in wheat caryopses under drought conditions. Biochemistry (Mosc) 2003, 68, 424–428, doi:10.1023/a:1023651913998.
- Swieiawska, B.; Jaworski, K.; Pawelek, A.; Grzegorzewska, W.; Szewczuk, P.; Szmidt-Jaworska, A. Molecular cloning and characterization of a novel adenylyl cyclase gene, HpAC1, involved in stress signaling in Hippeastrum x hybridum. Plant Physiol. Bioch. 2014, 80, 41–52, doi:10.1016/j.plaphy.2014.03.010.
- Ahn, S.J.; Shin, R.; Schachtman, D.P. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 2004, 134, 1135–1145, doi:10.1104/pp.103.034660.
- Han, M.; Wu, W.; Wu, W.H.; Wang, Y. Potassium transporter KUP7 is involved in K(+) acquisition and translocation in Arabidopsis root under K(+)-limited conditions. Mol. Plant 2016, 9, 437–446, doi:10.1016/j.molp.2016.01.012.
- Kohler, C.; Merkle, T.; Neuhaus, G. Characterisation of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis thaliana. Plant J. 1999, 18, 97–104, doi:10.1046/j.1365-313x.1999.00422.x.
- Arazi, T.; Kaplan, B.; Fromm, H. A high-affinity calmodulin-binding site in a tobacco plasma-membrane channel protein coincides with a characteristic element of cyclic nucleotide-binding domains. Plant Mol. Biol. 2000, 42, 591–601, doi:10.1023/a:1006345302589.
- Hua, B.G.; Mercier, R.W.; Zielinski, R.E.; Berkowitz, G.A. Functional interaction of calmodulin with a plant cyclic nucleotide gated cation channel. Plant Physiol. Bioch. 2003, 41, 945–954, doi:10.1016/j.plaphy.2003.07.006.
- Leng, Q.; Mercier, R.W.; Yao, W.; Berkowitz, G.A. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999, 121, 753–761, doi:10.1104/pp.121.3.753.
- Kugler, A.; Kohler, B.; Palme, K.; Wolff, P.; Dietrich, P. Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol. 2009, 9, 140, doi:10.1186/1471-2229-9-140.
- Guo, K.M.; Babourina, O.; Christopher, D.A.; Borsics, T.; Rengel, Z. The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol. Plant 2008, 134, 499–507, doi:10.1111/j.1399-3054.2008.01157.x.
- Tunc-Ozdemir, M.; Tang, C.; Ishka, M.R.; Brown, E.; Groves, N.R.; Myers, C.T.; Rato, C.; Poulsen, L.R.; McDowell, S.; Miller, G.; et al., A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 2013, 161, 1010–1020, doi:10.1104/pp.112.206888.
- Finka, A.; Cuendet, A.F.; Maathuis, F.J.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012, 24, 3333–3348, doi:10.1105/tpc.112.095844.
- Witters, E.; Valcke, R.; van Onckelen, H. Cytoenzymological analysis of adenylyl cyclase activity and 3 ‘: 5 ‘-cAMP immunolocalization in chloroplasts of Nicotiana tabacum. New Phytol. 2005, 168, 99–107, doi:10.1111/j.1469-8137.2005.01476.x.
- Molchan, O.V.; Sokolovsky, S.G.; Volotovsky, I.D. The phytochrome control of the cAMP endogenous level in oat seedlings. Russ. J. Plant Physl. 2000, 47, 463–467.
- Pietrowska-Borek, M.; Nuc, K. Both cyclic-AMP and cyclic-GMP can act as regulators of the phenylpropanoid pathway in Arabidopsis thaliana seedlings. Plant Physiol. Bioch. 2013, 70, 142–149, doi:10.1016/j.plaphy.2013.05.029.
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007, 144, 1777–1785, doi:10.1104/pp.107.101436.
- de Pinto, M.C.; Locato, V.; Paradiso, A.; De Gara, L. Role of redox homeostasis in thermo-tolerance under a climate change scenario. Ann. Bot. 2015, 116, 487–496, doi:10.1093/aob/mcv071.
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45, doi:10.1126/scisignal.2000448.
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–360, doi:10.1016/j.tplants.2014.06.013.
- Steinhorst, L.; Kudla, J. Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol. 2013, 163, 471–485, doi:10.1104/pp.113.222950.
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257, doi:10.1093/jxb/ert430.
- Pottosin, I.; Velarde-Buendia, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: Implications for plant adaptive responses. J. Exp. Bot. 2014, 65, 1271–1283, doi:10.1093/jxb/ert423.
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445, doi:10.1016/s1369-5266(03)00085-2.
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant 2008, 133, 651–669, doi:10.1111/j.1399-3054.2007.01008.x.
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329, doi:10.1038/nature05286.
- Wu, Y.; Zhou, J.M. Receptor-like kinases in plant innate immunity. J. Integr. Plant Biol. 2013, 55, 1271–1286, doi:10.1111/jipb.12123.
- Tang, D.Z.; Wang, G.X.; Zhou, J.M. Receptor kinases in plant-pathogen interactions: More than pattern recognition. Plant Cell 2017, 29, 618–637, doi:10.1105/tpc.16.00891.
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552, doi:10.1038/nri.2016.77.
- Cui, H.T.; Tsuda, K.; Parker, J.E. Effector-Triggered Immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511, doi:10.1146/annurev-arplant-050213-040012.
- Wang, W.; Feng, B.M.; Zhou, J.M.; Tang, D.Z. Plant immune signaling: Advancing on two frontiers. J. Integr. Plant Biol. 2020, 62, 2–24, doi:10.1111/jipb.12898.
- Kurosaki, F.; Nishi, A. Stimulation of calcium influx and calcium cascade by cyclic AMP in cultured carrot cells. Arch. Biochem. Biophys. 1993, 302, 144–151, doi:10.1006/abbi.1993.1192.
- Cooke, C.J.; Smith, C.J.; Walton, T.J.; Newton, R.P. Evidence that cyclic-AMP is involved in the hypersensitive response of Medicago-sativa to a fungal elicitor. Phytochemistry 1994, 35, 889–895, doi:10.1016/S0031-9422(00)90633-2.
- Zhao, J.; Guo, Y.Q.; Fujita, K.; Sakai, K. Involvement of cAMP signaling in elicitor-induced phytoalexin accumulation in Cupressus lusitanica cell cultures. New Phytol. 2004, 161, 723–733, doi:10.1111/j.1469-8137.2004.00976.x.
- Jiang, J.; Fan, L.W.; Wu, W.H. Evidences for involvement of endogenous cAMP in Arabidopsis defense responses to Verticillium toxins. Cell Res. 2005, 15, 585–592, doi:10.1038/sj.cr.7290328.
- Ma, W.; Qi, Z.; Smigel, A.; Walker, R.K.; Verma, R.; Berkowitz, G.A. Ca2+, cAMP, and transduction of non-self perception during plant immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 20995–21000, doi:10.1073/pnas.0905831106.
- Lomovatskaya, L.A.; Kuzakova, O.V.; Romanenko, A.S.; Goncharova, A.M. Activities of adenylate cyclase and changes in cAMP concentration in root cells of pea seedlings infected with mutualists and phytopathogens. Russ. J. Plant Physiol. 2018, 65, 588–597, doi:10.1134/S1021443718030056.
- Kurosaki, F.; Tsurusawa, Y.; Nishi, A. The elicitation of phytoalexins by Ca2+ and cyclic AMP in carrot cells. Phytochemistry, 1987, 26, 1919–1923, doi:10.1016/S0031-9422(00)81729-X.
- Mauch-Mani, B.; Slusarenko, A.J. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 1996, 8, 203–212, doi:10.1105/tpc.8.2.203.
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538, doi:10.1104/pp.110.157370.
- Shine, M.B.; Yang, J.W.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.Q.; Navarre, D.; Ghabrial, S.; Kachroo, P.; Kachroo, A. Cooperative functioning between phenylalanine ammonia lyase and isochorismate synthase activities contributes to salicylic acid biosynthesis in soybean. New Phytol. 2016, 212, 627–636, doi:10.1111/nph.14078.
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378, doi:10.1104/pp.106.079467.
- Torres, M.A, ROS in biotic interactions. Physiol. Plant 2010, 138, 414–429, doi:10.1111/j.1399-3054.2009.01326.x.
- Qi, J.S.; Wang, J.L.; Gong, Z.Z.; Zhou, J.M., Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol.2017, 38, 92–100, doi:10.1016/j.pbi.2017.04.022.
- Bolwell, G.P. Role of active oxygen species and NO in plant defence responses. Curr. Opin. Plant Biol. 1999, 2, 287–294, doi:10.1016/S1369-5266(99)80051-X.
- Bindschedler, L.V.; Minibayeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early signalling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol. 2001, 151, 185–194, doi:10.1046/j.1469-8137.2001.00170.x.
- Davies, D.R.; Bindschedler, L.V.; Strickland, T.S.; Bolwell, GP. Production of reactive oxygen species in Arabidopsis thaliana cell suspension cultures in response to an elicitor from Fusarium oxysporum: Implications for basal resistance. J. Exp. Bot. 2006, 57, 1817–1827, doi:10.1093/jxb/erj216.
- Clough, S.J.; Fengler, K.A.; Yu, I.C.; Lippok, B.; Smith, R.K.; Bent, A.F. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 2000, 97, 9323–9328, doi:10.1073/pnas.150005697.
- Balague, C.; Lin, B.Q.; Alcon, C.; Flottes, G.; Malmstrom, S.; Kohler, C.; Neuhaus, G.; Pelletier, G.; Gaymard, F.; Roby, D. HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 2003, 15, 365–379, doi:10.1105/tpc.006999.
- Yoshioka, K.; Moeder, W.; Kang, H.G.; Kachroo, P.; Masmoudi, K.; Berkowitz, G.; Klessig, D.F. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 2006, 18, 747–763, doi:10.1105/tpc.105.038786.
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. Death don’t have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 1996, 8, 1793–1807.doi:10.1105/tpc.8.10.1793.
- Grant, M.; Brown, I.; Adams, S.; Knight, M.; Ainslie, A.; Mansfield, J. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000, 23, 441–450, doi:10.1046/j.1365-313x.2000.00804.x.
- Lecourieux, D.; Raneva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phyto.l 2006, 171, 249–269, doi:10.1111/j.1469-8137.2006.01777.x.
- Lamotte, O.; Courtois, C.; Dobrowolska, G.; Besson, A.; Pugin, A.; Wendehenne, D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic. Bio. Med. 2006, 40, 1369–1376, doi:10.1016/j.freeradbiomed.2005.12.006.
- Ali, R.; Ma, W.; Lemtiri-Chlieh, F.; Tsaltas, D.; Leng, Q.; von Bodman, S.; Berkowitz, G.A. Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 2007, 19, 1081–1095, doi:10.1105/tpc.106.045096.
- Delledonne, M.; Xia, Y.J.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588, doi:10.1038/29087.
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 10328–10333, doi:10.1073/pnas.95.17.10328.
- de Jong, C.F.; Laxalt, A.M.; Bargmann, B.O.R.; de Wit, P.J.G.M.; Joosten, M.H.A.J.; Munnik, T. Phosphatidic acid accumulation is an early response in the Cf-4/Avr4 interaction. Plant J. 2004, 39, 1–12, doi:10.1111/j.1365-313X.2004.02110.x.
- Andersson, M.X.; Kourtchenko, O.; Dangl, J.L.; Mackey, D.; Ellerstrom, M. Phospholipase-dependent signalling during the AvrRpm1- and AvrRpt2-induced disease resistance responses in Arabidopsis thaliana. Plant J. 2006, 47, 947–959, doi:10.1111/j.1365-313X.2006.02844.x.
- D’Ambrosio, J.M.; Couto, D.; Fabro, G.; Scuffi, D.; Lamattina, L.; Munnik, T.; Andersson, M.X.; Alvarez, M.E.; Zipfel, C.; Laxalt, A.M. Phospholipase C2 affects MAMP-triggered immunity by modulating ROS production. Plant Physiol. 2017, 175, 970–981, doi:10.1104/pp.17.00173.
- Yang, T.B.; Poovaiah, B.W. A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J. Biol. Chem. 2002, 277, 45049–45058, doi:10.1074/jbc.M207941200.
- Du, L.Q.; Ali, G.S.; Simons, K.A.; Hou, J.G.; Yang, T.B.; Reddy, A.S.N.; Poovaiah, B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158, doi:10.1038/nature07612.
- Zhou, W.B.; Freed, C.R. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J. Biol. Chem. 2005, 280, 43150–43158, doi:10.1074/jbc.M507124200.
- Wei, H.R.; Persson, S.; Mehta, T.; Srinivasasainagendra, V.; Chen, L.; Page, G.P.; Somerville, C.; Loraine, A. Transcriptional coordination of the metabolic network in Arabidopsis. Plant Physiol. 2006, 142, 762–774, doi:10.1104/pp.106.080358.
- Shirasu, K. The HSP90-SGT1 Chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 2009, 60, 139–164, doi:10.1146/annurev.arPlant59.032607.092906.
- Yadeta, K.A.; Elmore, J.M.; Creer, A.Y.; Feng, B.M.; Franco, J.Y.; Rufian, J.S.; He, P.; Phinney, B.; Coaker, G. A Cysteine-rich protein kinase associates with a membrane immune complex and the cysteine residues are required for cell death. Plant Physiol. 2017, 173, 771–787, doi:10.1104/pp.16.01404.
- Ito, M.; Takahashi, H.; Sawasaki, T.; Ohnishi, K.; Hikichi, Y.; Kiba, A. Novel type of adenylyl cyclase participates in tabtoxinine-beta-lactam-induced cell death and occurrence of wildfire disease in Nicotiana benthamiana. Plant Signal. Behav. 2014, 9, 27420, doi:10.4161/psb.27420.
- Rich, T.C.; Webb, K.J.; Leavesley, S.J. Can we decipher the information content contained within cyclic nucleotide signals? J. Gen. Physiol. 2014, 143, 17–27, doi:10.1085/jgp.201311095.
- Wheeler, J.I.; Freihat, L.; Irving, H.R. A cyclic nucleotide sensitive promoter reporter system suitable for bacteria and plant cells. BMC Biotechnol. 2013, 13, 97, doi:10.1186/1472-6750-13-97.
- Jiang, J.Y.; Falcone, J.L.; Curci, S.; Hofer, A.M. Interrogating cyclic AMP signaling using optical approaches. Cell Calcium 2017, 64, 47–56, doi:10.1016/j.ceca.2017.02.010.
- Klausen, C.; Kaiser, F.; Stuven, B.; Hansen, J.N.; Wachten, D. Elucidating cyclic AMP signaling in subcellular domains with optogenetic tools and fluorescent biosensors. Biochem. Soc. Trans. 2019, 47, 1733–1747, doi:10.1042/BST20190246.
- Rahman, N.; Buck, J.; Levin, L.R. pH sensing via bicarbonate-regulated “soluble” adenylyl cyclase (sAC). Front. Physiol. 2013, 4, 343, doi:10.3389/fphys.2013.00343.
- Paramonov, V.M.; Mamaeva, V.; Sahlgren, C.; Rivero-Muller, A. Genetically-encoded tools for cAMP probing and modulation in living systems. Front. Pharmacol. 2015, 6, 196, doi:10.3389/fphar.2015.00196.
