Pyrolysis process has been considered to be an efficient approach for valorization of lignocellulosic biomass into bio-oil and value-added chemicals. Bio-oil refers to biomass pyrolysis liquid, which contains alkanes, aromatic compounds, phenol derivatives, and small amounts of ketone, ester, ether, amine, and alcohol. Lignocellulosic biomass is a renewable and sustainable energy resource for carbon that is readily available in the environment. This review article provides an outline of the pyrolysis process including pretreatment of biomass, pyrolysis mechanism, and process products upgrading. The pretreatment processes for biomass are reviewed including physical and chemical processes. In addition, the gaps in research and recommendations for improving the pretreatment processes are highlighted. Furthermore, the effect of feedstock characterization, operating parameters, and types of biomass on the performance of the pyrolysis process are explained. Recent progress in the identification of the mechanism of the pyrolysis process is addressed with some recommendations for future work. In addition, the article critically provides insight into process upgrading via several approaches specifically using catalytic upgrading. In spite of the current catalytic achievements of catalytic pyrolysis for providing high-quality bio-oil, the production yield has simultaneously dropped. This article explains the current drawbacks of catalytic approaches while suggesting alternative methodologies that could possibly improve the deoxygenation of bio-oil while maintaining high production yield.
- Biomass
- Bio-oil
- Characterisation
- Extraction
- Pretreatment
- catalytic upgrading
- biofuel
- pyrolysis
- Definition
Pyrolysis process has been considered to be an efficient approach for the valorization of lignocellulosic biomass into bio-oil and value-added chemicals. Bio-oil refers to biomass pyrolysis liquid, which contains alkanes, aromatic compounds, phenol derivatives, and small amounts of ketone, ester, ether, amine, and alcohol.
- Introduction
The total world energy consumption is increasing at a steep rate due to global industrialization, and it is expected to reach 28% by 2040. Currently, fossil fuels are considered to be the main source of energy which release toxic and greenhouse gases, particulates, and pollutants leading to significant environmental impacts. Under the European Union (EU) 2030 energy framework and climate actions, a 27% increase in the share of renewable fuels and 40% reduction in greenhouse gases are targeted by 2030 [1]. Accordingly, to meet the rising energy demand while showing concern for the environmental aspects, alternative sustainable and environmentally benign fuels should be developed. In this regard, research on novel approaches for renewable engineered fuels with low emission and high heating value is crucial. Furthermore, it is essential to consider relevant ethical consideration by excluding any feedstocks and compounds used for the food industry, i.e., edible vegetable oils and fresh crops [2,3]. The source of carbon in biomass results from the photosynthesis process as part of the plant growing cycle where carbon dioxide is consumed. Biomass is considered to be the largest available carbon source that is renewable and sustainable for biofuels production. Biomass is abundant, renewable, and inexpensive [4], and it supplies about 14% of the world’s yearly energy consumption. Biomass refers to organic materials that are produced from plants, animals, and other living organisms, i.e., microorganisms [5].
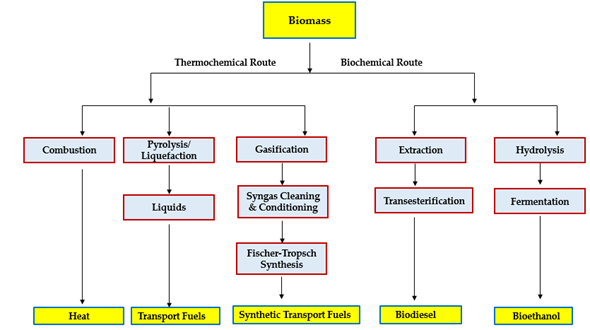
Biomass valorization into biofuels and value-added chemicals has been achieved via different routes. On the one hand, for instance, vegetable oils are used to produce biodiesel, green diesel, and value-added chemicals through several reactions including transesterification [6], esterification [7], hydrogenation, hydrolysis, etc. Furthermore, sugary plants have been used to produce bioethanol via the fermentation process. On the other hand, biomass has been directly converted into biofuels via thermochemical processes including pyrolysis, gasification, liquefaction, etc. [8–11]. Generally, biomass is categorized based on the feedstock into three categories namely first, second, and third generations. First-generation biomass refers to the edible and virgin biomass that can be used in food industries such as vegetable oils and fresh crops. The competition between energy and the food industry for first-generation biomass has led to food insecurity concerns. Accordingly, several constraints have been developed to prevent using first-generation feedstock as an energy resource [12,13]. As a result, the second-generation feedstock of biomass has been defined as non-edible plant resources and waste biomass including waste food, waste vegetable oils, lignocellulosic biomass, etc. Research on valorizing second-generation feedstock has been widely reported in the last few years to produce biodiesel, bioethanol, biogas, bio-oil, etc. Finally, third-generation feedstock has been recently reported as the algal and microorganism’s feedstock. The main advantage of the third-generation feedstock is the rapid growth rate, the requirement of a small area to grow, and the easily controlled growth conditions [14]. A simplified schematic for the conversion routes of biomass is presented in Figure 1.
Figure 1. A simplified schematic illustration of two main biofuel production pathways (adapted from [15]).
Thermochemical conversion technology produces a wide range of products including gaseous, condensable vapours and solids. The condensable vapours of the process are mainly obtained as bio-oil, where they can be upgraded to biofuels and value-added chemicals. Unlike biological processes that can convert only limited components of the biomass, thermochemical processes are capable of converting all the carbon in the feedstock [16]. Open-air combustion has been considered to be the oldest technology used for biomass valorization for heating benefits. This technology is still the dominant process for heating from biomass in numerous parts of the world. Since then, several thermochemical technologies have been developed to overcome the disadvantages and limitation of open-air combustion. The main development in thermochemical technology is the aim to produce different biofuels and value-added chemicals from biomass. Charcoal was the first reported biofuel produced from wood, which was considered to be the spark for the current progress in thermochemical technologies. As compared with petroleum coal, charcoal has higher oxygen content which reduces the required excess air for combustion. Furthermore, most of the carbons in the biomass are bounded to oxygen or hydrogen (organic carbon), which make them more volatile in relation to elementary carbon. Hence, the conversion of biomass volatile compounds can be achieved between 200 and 600 °C and above 800 °C for non-volatile compounds [17].
Recently, several technologies have been reported for thermochemical valorization of biomass including torrefaction, hydrothermal liquefaction, pyrolysis, and gasification which can readily convert biomass into bio-oil, syngas, heat, and charcoal. Torrefaction has been developed as a pretreatment process for biomass to improve its fuel and physicochemical properties. The process is carried out between 200 and 300 °C with a slow heating rate lower than 50 °C /min. The main advantages of the torrefaction process are that it reduces the moisture content, hydrophobicity, and volatile matter of biomass. During torrefaction, hydrophobicity is reduced due to the carboxylic groups’ degradation. It also improves the heating values and grindability of biomass [18]. Hydrothermal liquefaction is defined as the process of valorizing biomass into solid, liquid, and gaseous products in sub- and supercritical water, solvents, and catalysts. The process operates between 250 and 380 °C and between 4 and 230 bar. The main product produced from hydrothermal liquefaction is bio-oil which is similar to petroleum crude oil with a high mixture of oxygenated compounds. The quality of bio-oil is characterized by viscosity, H/C and O/C ratios, as well as density and heating values. Several biofuels and chemicals can be extracted from bio-oil based on the implemented downstream process. Furthermore, co-liquefaction of different types of biomass has been recently reported to increase the yield of bio-oil [19].
Pyrolysis is the process of thermal decomposition of biomass in the absence of oxygen to produce bio-oil, char, and gaseous product. It is considered to be a promising approach for biomass valorization in a short period of time yielding up to 78 wt% of bio-oil (based on dry biomass). The pyrolysis process operates between 400–650 °C. According to product preferences, the process could be classified into slow and fast pyrolysis in terms of the heating rate. On the one hand, slow pyrolysis is the process that favors producing solid biochar and the process performs up to a few hours. On the other hand, fast pyrolysis is the process for enhancing the production of bio-oil (condensable vapours) and the process operates at a very high heating rate reaching the process temperature in a few seconds. Furthermore, fast pyrolysis uses a maximum particle size of feedstock of 2 mm [5]. Finally, gasification is a process where biomass is converted at a relatively high temperature (<700 °C) with incomplete combustion in a controlled oxygen/steam environment. The process results in syngas (main product), condensable vapours, and char. Syngas or synthesis gas is a mixture that is comprised of carbon monoxide, carbon dioxide, and hydrogen. Compared with conventional incineration (combustion), gasification is a more efficient process for electricity generation because syngas can be easily utilized for electricity by using gas engines, gas turbines, or fuel cells. Moreover, the gasification process is also superior to biological methods as it can convert all types of biomass unlike fermentation. In summary, the main advantages of gasification are the conversion of the entire carbon content in the biomass, and production of valuable fuels, i.e., hydrogen, bio-oil, and lower CO2 emission [20].
- Pyrolysis products and methods
The pyrolysis products result from the primary decomposition of biomass followed by secondary reactions of condensable products into low molecular weight gases and char [30]. In general, large hydrocarbon molecules of biomass material are broken into smaller hydrocarbons. Bio-oil refers to biomass pyrolysis liquid, which contains alkanes, aromatic compounds, phenol derivatives, and small amounts of ketone, ester, ether, amine, and alcohol. The chemical compositions of bio-oil are defined by several factors including the type of biomass, process parameters (temperature, heating rate, and residence time), as well as condensation process (condensing technique and cooling rate). However, the pyrolysis process requires accurate control of temperature and short residence time (less than 3 s) to achieve a high yield of bio-oil [31]. Depending on the rate of applied heat, and the preferred types of the product (gas, solid, and liquid), pyrolysis can be classified into three different variations, i.e., fast, intermediate, and slow pyrolysis. The mode and the operating conditions of pyrolysis can affect the relative proportions of the gas, liquid, and solid products [32]. The fast pyrolysis process can be achieved at a high temperature in which biomass is fast heated in the absence of oxygen by introducing an inert gas to the reaction and at a high temperature of 400–600 °C; the feedstock reaches the peak temperature before the decomposition process takes place [33]. This process requires that the feedstock is prepared as small particle sizes for a rapid char removal design. Particle size is defined as the average diameter, in microns, of solid materials such as biomass. Slow pyrolysis produces some gas and solid charcoal and uses a low heating rate, with a long vapour residence time and typically a lower temperature than when fast pyrolysis is applied. The target product for slow pyrolysis is often char [32]. An intermediate pyrolysis process can be conducted at temperatures between 500 and 650 °C in a fixed bed pyrolysis reactor.
The fast pyrolysis of biomass is the commonly preferred method because of the fast rate of reaction and higher yields of liquid products. In the last ten years, numerous researches have studied the fast pyrolysis process focusing on reducing the water and oxygen contents, acidity, and viscosity of bio-oil, through different upgrading methods. In addition, further, development was achieved by optimizing the reaction conditions, and the improvement of accurate models that correspond to the kinetics of biomass thermal degradation for the production of bio-oil. Many authors have investigated the composition and distribution of three main products (liquid, solid, and gas) of biomass pyrolysis produced from different types of biomass with different operating parameters and different reactor configurations. Temperature is the most important parameter to be considered in the pyrolysis process as it directly affects the production of bio-oil. At higher temperatures, the char yield decreases significantly. This happens because of the primary decomposition of biomass at high temperatures and the formation of char is by secondary thermal decomposition [33,34].
- Pyrolysis Products
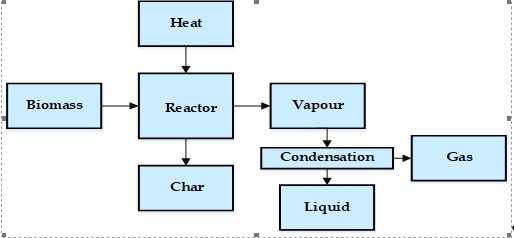
The most valuable product of pyrolysis is the volatile product, which after the condensation process, is converted to a liquid fraction of the pyrolysis process, known as bio-oil. This bio-oil is a complex mix of hundreds of organic compounds, containing alcohols, ketones, aldehydes, phenols, and oligomers [101]. In addition, the pyrolysis process produces a solid by-product, which is called ash. Heavy metals can be present in the solid product of the pyrolysis process that was added to the biomass during collecting and processing of the raw materials. The characterization of bio-oil is essential for defining reactor design parameters, defining kinetic models, upgrading, and commercialization [101]. The distribution of the product of pyrolysis depends on the design of the pyrolysis reactor, as well as the physical and chemical characterization of the raw materials and operating parameters. In Figure 16, the reaction pathway of the pyrolysis process is presented.
Figure 2. Reaction pathway of the pyrolysis process.
4.1. Bio-Oil
The produced bio-oil from the pyrolysis process is yellowish to brownish liquid accompanied with a pungent odor. Bio-oil is considered to be a complex mixture of compounds with very limited application as crude bio-oil because of its poor properties. Crude bio-oil could only be used as a fuel for boilers, but not for engines due to its low heating value, water content, and acidity. The physicochemical properties of the crude bio-oil produced from woody biomass is summarized in Table 3.
Table 1. Physicochemical properties of bio-oil produced from woody biomass [102].
|
Physical Property |
Typical Value |
|
Moisture content |
25% |
|
pH |
2.5 |
|
Elemental analysis C H O N |
56% 6% 38% 0–0.1% |
|
HHV (Higher heating values) as produced |
17 MJ/kg |
|
Viscosity (40 °C and 25% water) |
40–100 mPa s |
|
Solids (char) |
0.1% |
|
Vacuum distillation residue |
up to 50% |
The biomass type and the operating conditions of pyrolysis significantly affect the chemical composition of the produced bio-oil. Generally, water content represents 15–35% of the bio-oil weight. The existence of water is inevitable as per the moisture in the feedstock and specific reactions, i.e., dehydration, that occurs during the thermal decomposition of biomass [103]. Water content in bio-oil is considered to be a disadvantage as it lowers the heating value and enhances phase separation. It also contributes by lowering the pH of the bio-oil. Both water content and oxygen are the main reasons for the low heating value (LHV) of bio-oil [104,105].
Bio-oil characterization could not be applied for the crude product as it combines hundreds of different species and compounds. Hence, several downstream processes for crude bio-oil have been reported including distillation, emulsification, adsorption, and solvent extraction. From the separation processes, solvent extraction has been proven to be an efficient method for the separation of chemical organic families from crude bio-oil [106]. Different types of solvents have been reported including methanol, butanone, dichloromethane, hexane, diethyl-ether, acetone, ethyl acetate, etc. Furthermore, a mixture of solvents has been reported as an efficient method for fractionation of various compounds from bio-oil which have been characterized using gas chromatography-mass spectrometry (GC-MS) [107]. The basic principle applied in solvent extraction is the polarity of the solvents and the extracted compounds. The main advantage of solvent extraction is the separation of similar chemical compounds under the same chemical family together using a specific solvent [9]. The composition of bio-oil has been identified based on the grouping method of certain extracted chemical families. More than 10 chemical families have been fractionated from bio-oil including aromatics, ketones, phenolics, sugars, ethers, alcohols, esters, furans, etc. [9]. It has also been reported that most of the bio-oil extracted compounds are oxygenated with high polarity and high solubility in water [108]. Bio-oil composition depends on numerous variables including biomass type, heating rate, particle size of feedstock, and residence time. Particle size is a noteworthy parameter that affects the composition where small particle size enhances the uniform heat transfer within the particles, and hence results in high bio-oil yield [38].
Bio-oil yield increases as the pyrolysis temperature is increased up to a certain maximum temperature, after which the yield drops as the temperature is further increased [109]. However, it has been reported that higher cellulose content in the feedstock enhances the yields of bio-oil [110]. Thus, the bio-oil yield can be influenced by parameters such as type of biomass and operating conditions. The most common feedstock that have been found to produce bio-oil from pyrolysis and hydrothermal processes were rice husk [111], cotton stalk [112], oil palm, and palm kernel shell [77]. In Table 4, various types of biomass with their respective produced bio-oil yield are compared. As stated, the type of biomass has a significant effect in the pyrolysis process and bio-oil production. Different types of feedstocks have different moisture contents, ash contents, higher heating values (HHV), and elemental compositions (N, O, S, H, C). In Table 5, elemental compositions of different types of biomass are compared.
Table 2. Various types of biomass with their respective produced bio-oil yield.
|
Biomass Type |
Type of Reactor |
T (°C) |
Bio-Oil Yield wt% |
Reference |
|
Sugarcane bagasse |
Fluidized bed |
500 |
74.0 |
[113] |
|
Sawdust |
Fluidized bed |
500 |
76.0 |
[113] |
|
Banana rachis |
Fluidized bed |
500 |
28.0 |
[113] |
|
Corncob |
Fluidized bed |
550 |
56.8 |
[114] |
|
Rice husks |
Fluidized bed |
450 |
60.0 |
[114] |
|
Cedar wood |
Quartz glass tube reactor |
550 |
46.8 |
[115] |
|
Poplar |
Spouted bed |
455 |
69.0 |
[116] |
|
Rice husk |
Spouted bed reactor |
450 |
70.0 |
[117] |
|
Palm kernel shell (PKS) |
Iconel batch |
390 |
38.5 |
[118] |
|
Empty fruit bunch (EFB), |
Iconel batch |
390 |
37.4 |
[118] |
|
Palm mesocarp fiber (PMF) |
Iconel batch |
390 |
34.3 |
[118] |
|
Sweet sorghum bagasse |
Fluidized bed |
500 |
43.5 |
[119] |
|
Blue-green algae blooms |
Fixed bed |
500 |
55.0 |
[120] |
|
Corncob |
Fluidized bed |
550 |
56.8 |
[121] |
|
Cotton Stalk |
Fluidized bed |
510 |
55.0 |
[122] |
Table 3. Elemental compositions (N, O, S, H, C) different types of biomass.
|
Biomass Type |
C (wt%) |
H (wt%) |
N (wt%) |
S (wt%) |
O (wt%) |
Ash (wt%) |
HHV (MJ/kg) |
Reference |
|
Sugarcane bagasse |
45.5 |
6.0 |
45.2 |
- |
0.15 |
3.2 |
18.7 |
[123] |
|
Coconut shell |
50.2 |
5.7 |
43.4 |
- |
- |
0.71 |
20.5 |
[123] |
|
Cotton stalk |
47.1 |
4.6 |
1.2 |
- |
42.1 |
5.1 |
17.4 |
[124] |
|
Sunflower |
50.5 |
5.9 |
1.3 |
0.1 |
34.9 |
6.9 |
20.3 |
[125] |
|
Energy grass |
48.3 |
5.5 |
0.6 |
0.1 |
41.5 |
3.8 |
19.1 |
[125] |
|
Wood waste |
49.7 |
6.0 |
1.7 |
0.0 |
41.0 |
1.5 |
18.6 |
[125] |
|
Corncob |
49.0 |
5.4 |
0.4 |
- |
44.6 |
1.0 |
17.0 |
[126] |
|
Tea waste |
48.6 |
5.5 |
0.5 |
- |
39.5 |
1.4 |
17.1 |
[126] |
4.2. Biochar
The solid product of the pyrolysis process is biochar, which is the highly carbonaceous material and the carbon content is between 65–90% [127]. The characterization of biochar is defined by the type of biomass and the operating conditions of the pyrolysis process, which affect the carbon content of biochar. In recent years, there has been a great attraction to biochar in a number of environmental applications. One of the potential applications of biochar is its use as a C and N source in soil amendment [128,129] to improve the fertility of the soil and enhance the agricultural production. However, biochar has unique properties to remove pollutants from soil, water, and gas [130]. These unique properties include, for example, high adsorption capacity, high specific surface area, microporosity, and ion exchange capacity [130]. Biochar can be used as a contaminant remover (adsorbent) to remove the toxic pollutants from affected waters or soils. These abilities are based on a porous structure, oxygen functional groups, and a large surface area of biochar [131]. The presence of oxygen containing functional groups on the surface of the biochar helps in the reduction of heavy metal such as lead, nickel, cadmium, and copper in contaminated soils [132]. It has been reported that higher biochar yield was formed by pyrolysis of biomass with higher lignin content [133]. Furthermore, slow pyrolysis is more favored for biochar production. Biochar has high resistance to microbial decomposition. Hence it has high stability for long periods of time (1000 to 10,000 years), which helps in carbon sequestration [134,135]. Furthermore, a novel advanced application of biochar, attracting considerable interest in recent years, is the novel materials for supercapacitor electrodes [136]. Batteries and capacitors are known as energy storage systems. The unique features of supercapacitors which include high power, environment-friendly (organic electrodes), and long cycle life have attracted significant attention from researchers for improving the performance of supercapacitors over the last few years [137].
4.3. Pyrolytic Gas
The main gases produced in the pyrolysis of biomass are a mixture of H2, hydrocarbon gases (C1–C4), CO2, CO, and H2S [138]. The pyrolytic gases can be classified into three categories including incombustible gases (H2O and CO2), combustible gasses (CO and CH4), and N-containing gases (NH3 and HCN). A lower pyrolysis temperature results in lower yield of gases, whereas with an increase in temperature, the biomass undergoes further secondary reactions to form pyrolytic gases [66]. In addition, the use of zeolite catalyst for pyrolysis at 500 °C, increases the pyrolysis gas yield [139]. As revealed from the literature, the formation of CO2 mainly originates from decomposition reactions of carbonyl and carboxyl groups in biomass pyrolysis reaction, whereas the formation of CO mainly results from breaking of C-O-C and C = O bonds [140]. However, H2 mainly results from breaking of C-H groups and aromatics. However, CO and CO2 are dominant gaseous products at low temperatures and CH4 is a dominant product at high temperatures due to lignin depolarization reactions [141,142].
- Perspective
The valorization of lignocellulosic biomass via pyrolysis has been proven to be an efficient solution for the production of biofuels and value-added chemicals. Numerous studies have reported on experimental pyrolysis, kinetic studies, process simulation, and bio-oil characterization to realize the precise mechanism of pyrolysis. However, many challenges still need to be addressed including the difficulties in providing a systematic approach for biomass pretreatment. In spite of the achievements in studying the mechanisms for pyrolysis, defining an accurate mechanism for the pyrolysis process is still a challenge.
The physical pretreatment of lignocellulosic biomass has been comprehensively reviewed including particle size reduction, densification, and torrefaction. In addition, chemical pretreatment processes including acidic, alkaline, hydrothermal, steam explosion, and wet torrefaction have been highlighted. However, it should be noted that the literature lacks any application of ionic liquids pretreatment for biomass prior to pyrolysis, which might have favoured the impact of the efficiency of the process.
One of the main challenges of the pyrolysis process is the quality of the produced bio-oil, which generally has high oxygen and water content and could make crude bio-oil unsuitable for direct fueling in the existing engines. The development of a suitable upgrading method for oxygen and water removal would lead to the production of high-quality bio-oil to compete with petroleum fuels. This article has reviewed several upgrading technologies for bio-oil including supercritical fluids, solvent extraction, emulsification, hydrodeoxygenation, steam reforming, and catalytic approaches. The main challenge in most of the upgrading technologies is the inconsistency of the bio-oil yield. Thus, the key is to economically optimize the process to produce high-quality bio-oil with an acceptable yield. The removal process of oxygen using the in situ catalytic approach has been considered to be an efficient technology for bio-oil up-gradation via dehydration with zeolite catalyst, i.e., ZSM-5. In fact, dehydration enhances coke formation and produces a lower yield of high-quality bio-oil. However, decarboxylation would be a promising up-gradation approach that keeps the high yield of good quality bio-oil. The key for successful decarboxylation is the pre-upgrading of volatiles to inhibit the coke formation, and hence increase the bio-oil yield.
Research on upgrading the bio-oil product while maintaining high yield should be considered for future work. Alternative catalysts for zeolites including basic metal oxides, i.e., MgO and CaO should be extensively studied for deoxygenation of bio-oil. More efforts should address inorganic salt additives in the feedstock and carbon-based catalysts that could enhance the selectivity of the process and produce phenolic rich bio-oil. Catalytic co-pyrolysis could be considered to be an ideal solution that would enhance the deoxygenation reaction with a simultaneous high yield of bio-oil.
Finally, it is worth mentioning that the integration of pretreatment techniques with the catalytic pyrolysis would enhance the conversion of low-density oxygenated biomass into high-quality aliphatic and aromatic hydrocarbons. The process optimization techniques should be extensively applied in the pyrolysis process to minimize the process variables energy consumptions, i.e., temperature, pressure, catalyst loading, reactor configuration, particle size of biomass, while maintaining high-quality bio-oil and high yield of bio-oil.
This entry is adapted from the peer-reviewed paper 10.3390/pr8070799