Tannins are one of the most natural, non-toxic, and highly reactive aromatic biomolecules classified as polyphenols. The reactive phenolic compounds present in their chemical structure can be an alternative precursor for the preparation of several polymeric materials for applications in distinct industries: adhesives and coatings, leather tanning, wood protection, wine manufacture, animal feed industries, and recently also in the production of new porous materials (i.e., foams and gels). Among these new polymeric materials synthesized with tannins, organic and carbon gels have shown remarkable textural and physicochemical properties.
- tannin
- polyphenolic molecules
- sol-gel
- organic gel
- carbon gel
- hydrothermal carbonization
- porous materials
- pore structure
- biopolymer
- low-cost
1. The Chemistry of Tannins
1.1. Definition and Classification
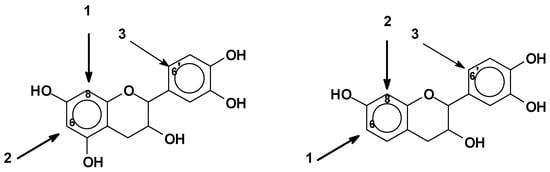
1.2. Condensed Tannins
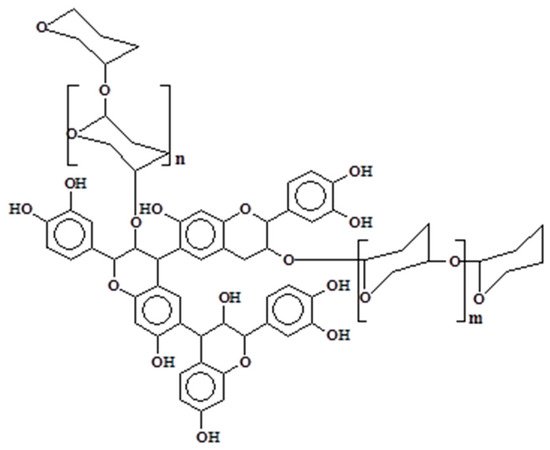
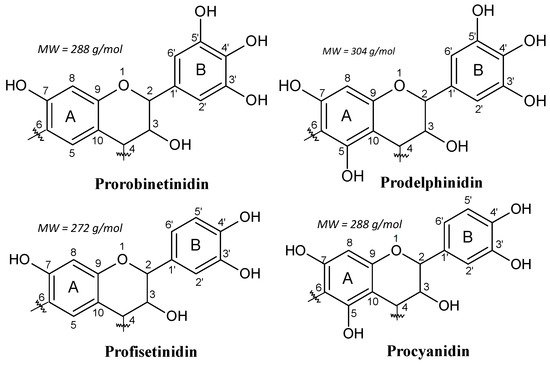
2. Tannin Gels
2.1. Tannin Gels Synthesis
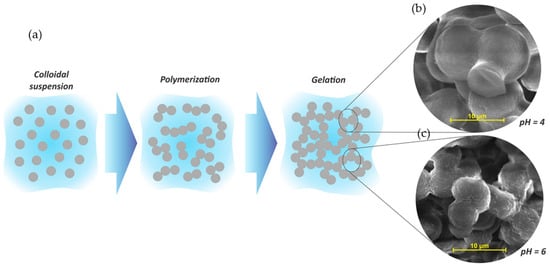
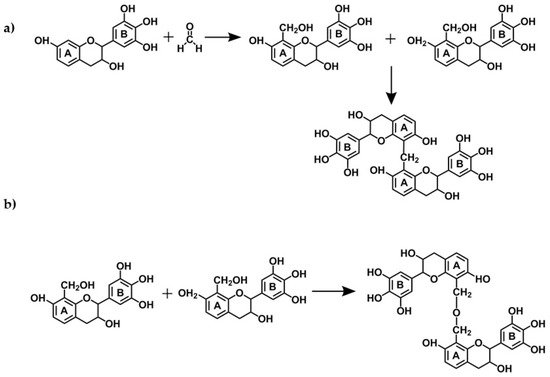
2.2. Mechanisms
2.2.1. Tannin-Formaldehyde-Systems
2.2.2. Influence of pH and Mass Fraction
2.2.3. Tannin-Soy-Formaldehyde Gels
2.3. Preparation Methods and Conditions
2.3.1. Hydrogels Formulations at Normal Conditions
2.3.2. Solvent Exchange
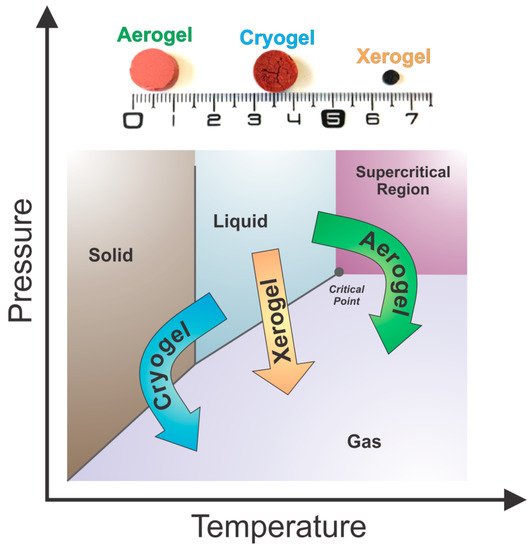
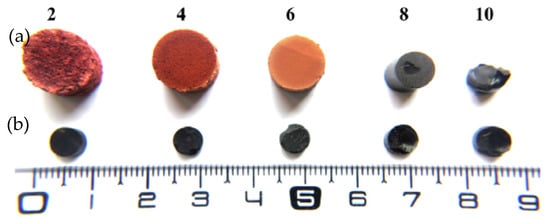
2.3.3. Drying Conditions and Final Physicochemical Properties of Tannin Gels
-
Subcritical drying: Gels are dried under atmospheric conditions to form xerogels.
-
Freeze-drying: Gels are dried at freezing conditions to produce cryogels.
-
Supercritical drying: Gels are dried at a critical point of a working fluid to produce aerogels.
| Precursors | Conditions | Main Findings | Ref. |
|---|---|---|---|
| Hydrogels Prepared Under Normal Conditions Organic Tannin Gels |
|||
| Tannin + formaldehyde (TF) | Hydrogels prepared at 85 °C for 120 h; Mass fraction of total solids (MFTS) of 4–40 wt.%; NaOH and p-toluene sulfonic acid were used to change the initial pH of the solution (4.3); pH = 2−10; Supercritical drying in CO2 |
Organic aerogels; SBET = 219–880 m2/g; Porosity = 40–97% The highest SBET (880 m2/g) and the highest micropore volume (0.28 cm3/g) were obtained for a gel prepared with MFTS of 10 wt.% and pH 6. The maximum mesopore volume (1.34 cm3/g) and the highest pore volume (21.5 cm3/g) were found for samples with a MFTS of 18 and 4 wt.% at pH 8 and 2, respectively. The transparency of gels was associated with the structure of the aquagel: larger nodules at low MFTS (˂ 18 wt.%) and low pH (especially 2) lead to larger clusters and pore volumes (21.5–4.9 cm3/g), reducing the transparency of the final gel. By increasing the pH (4–10) and MFTS (22–40 wt.%), the nodules were reduced, and the transparency tended to increase. Aerogels with MFTS greater than 22 wt.% with extremely high pH (10) resulted in a low porous material (0.48–1.01 cm3/g). The most diluted samples (4 and 6% of TF resin at pH 6 and 8, respectively) presented the highest shrinkage volumes (~ 87%) during drying step, which was caused by high capillary forces due to their low mechanical properties. These values are comparable to those of aerogels from natural biopolymer, such as cellulose (75 wt.%). The SBET values found for samples with MFTS lower than 20 wt.% were higher than those of any other bioresourced organic aerogel. |
[60] |
| Tannin + formaldehyde + Pluronic (TFP) | Hydrogels prepared at 85 °C for 120 h with Pluronic® F-127, using a mass ratio of T/P of 2; NaOH and p-toluene sulfonic acid were used to change the initial pH of the solution (4.9); pH = 2−10; Subcritical drying in two steps: air and 80 °C |
Organic xerogels; SBET = ˂ 1.5 m2/g; Porosity = 2–79% The surfactant P was employed as an additive to decrease the surface tension of the aqueous system inner porosity and consequently mitigate the shrinkage during drying step. The system TFP presented a correlation between porosity characteristics (pore volume and centered peak pore size measured through mercury porosimetry) and pH. Thus, pH played a crucial role in pluronic-tannin gels preparation. The xerogels prepared at pH 2–7 presented a bulk density comparable to those of aerogels (0.30–0.58 cm3/g). Such a system demonstrated an interesting and low-cost technique for preparing porous gels with tailored porosity on a large scale. The final xerogels presented unimodal (macroporosity) or bimodal (macro-mesoporosity) porosity. |
[61] |
| Tannin + soy + formaldehyde (TSF) |
Hydrogels prepared at 85 °C for 120 h; Proportions of T/S resin of 30 and 70 wt.% (on a dry basis), respectively; NaOH in pellets and H3PO4 (85%) were used to change the initial pH of the solution (8.2); pH 5.5−9; Mass ratio of total solids 14 wt.%; Supercritical drying in CO2 |
Organic aerogels; SBET = 384–478 m2/g; Porosity = 84–88% The pH of the initial solution had a significant impact on gelation: the lowest gelation time, which happened at pH 6, produced the highest SBET (478 m2/g) and micropore volume (0.15 cm3/g). Curiously, this aerogel also presented the highest mesopore volume (2.29 cm3/g). A correlation between SBET and gelation time was noticed. The TSF aerogels were based on a filamentous structure, which is comparable to cellulosic and lignocellulosic aerogels. Organic aerogels are natural at 91%, and mesopore volumes (1.72–2.29 cm3/g) are among the greatest ever volumes reported for aerogels of natural origin, considering the same density (0.19–0.25 g/cm3). |
[54] |
| Tannin + lignin + formaldehyde (TLF) | Hydrogels prepared at 85 °C for 120 h; Mass ratio of T/L of 0.11–1 (on a dry basis); Mass ratio of ((L + T)/F) of 0.83–2.5 (on a dry basis); Initial pH of 10; Supercritical drying in CO2 |
Organic aerogels; SBET = 50–550 m2/g; Porosity = 72%–87% The SBET of TLF aerogels was mostly related to mesopores volumes (0.2–1.4 g/cm3), since micropores were extremely low (0.01–0.02 cm3/g). The highest SBET values were found for TLF gels at mass fractions of T/L=1 and (L + T)/F = 1.25, demonstrating that tannin could be replaced by lignin at 50 wt.%. Besides, there is an optimal quantity of formaldehyde that can be used to produce highly porous aerogels. Organic aerogels were synthesized using up to 71% of natural precursor. |
[78] |
| Tannin + resorcinol + formaldehyde + sodium dodecyl sulfate (TRFSDS) | Preparation in oven: Hydrogels prepared at 85 °C for 72 h (gelation) and 48 h (curing); Preparation in a microwave: 85 °C for 3 h (gelation and curing) and 1–2 h (drying); NaOH and SDS were used as catalyst and surfactant, respectively; Initial pH of 5.5; SDS amount of 5 wt.%; Mass ratio of (T + R)/F of 1.2; MFTS of 25 wt.%; T was used in different proportions (0–100 wt.%) to replace R |
Organic xerogels; Porosity = 20–85% The synthesis of tannin xerogels in a microwave oven allowed a reduction in time of 95%, including the time from sol-gel reaction until complete drying. Resorcinol could be replaced by tannin in the production of organic xerogels. However, the use of surfactant is always required because the structure collapses in the presence of large quantities of tannin (>25 wt.%). Materials with high proportion of tannin (75 wt.%) were produced with very small difference of porosity through conventional and microwave synthesis conditions (77% and 62%, respectively). |
[124] |
| Carbon tannin gels | |||
| Tannin microspheres (TM) | Microspheres prepared at 80 °C for 1 h stirred at 200–1200 rpm; Mixture of surfactant (SPAN 80) (0–10%) and sunflower oil; Initial pH of 4.3; MFTS of 40 wt.%; Carbonization: 900 °C for 2 h; Heating rate of 2 °C/min |
Carbon xerogels; SBET = 7–666 m2/g Microspheres were prepared by inverse emulsion polymerization in sunflower oil, followed by subcritical drying and carbonization. Higher stirring speed produced microspheres with low average size due to the shear rate that lead particles (microspheres) at smaller sizes. At the same time, smaller microspheres were produced by increasing the surfactant content and promoting greater contact between oil and the aqueous phase. This process formed small drops of resin that became microspheres after gelation. However, there was a limit amount of surfactant and speed stirring to produce small microspheres of 5 wt.% and 500 rpm, respectively. The mean deviation decreased with the increasing speed stirring (with or without surfactant) and the final microspheres presented narrow micropore size distribution centered at 0.4–0.5 nm. |
[125] |
| Tannin + formaldehyde (TF) | Hydrogels prepared at 85 °C for 120 h; pH from 3.3 to 8.3; Supercritical drying in acetone; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min |
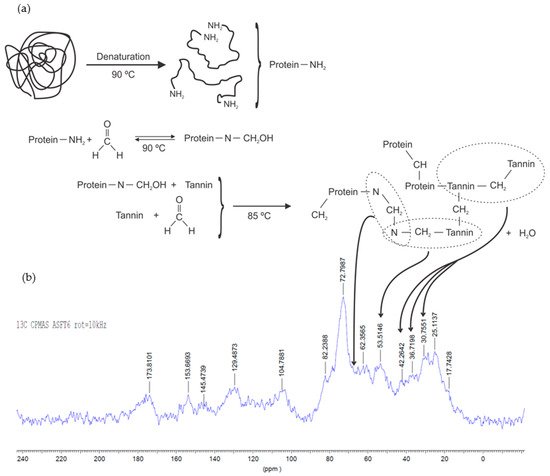
Carbon aerogels; SBET = 580–720 m2/g; Porosity = 75–99% Carbon aerogels based on tannin-formaldehyde solutions were prepared in a broad pH range. The microstructure of gels presented the typical nodular shape of phenolic aerogels, where their size was determined by the initial pH of the solution. The polymerization reaction was performed with methylene or methylol bridges, as showed by 13C NMR analysis. The final material presented high mesopore fraction (57–78%). The synthesized TF aerogels were five times cheaper than resorcinol-formaldehyde aerogels. |
[77] |
| Tannin + resorcinol + formaldehyde (TRF) | Hydrogels prepared at 50 °C for 10–160 min (depending on gelation time); Acetic acid or orthophosphoric acid were used to change the initial pH of the solution (8.15); pH = 2−8; Freeze-drying; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min |
Carbon cryogels; SBET = 30–650 m2/g; Porosity = 32–80% Low cost cryogels were produced by replacing two third of resorcinol by tannin, which resulted in a reduction of the final cost of the gel by a factor of 2. Also, freeze-drying represents a low-cost drying method compared to supercritical drying. The SBET and porosity decreased by increasing pH. Furthermore, SBET values were comparable to resorcinol-formaldehyde cryogels and phenol-formaldehyde aerogels, which are gel materials based on more expensive precursors. |
[53] |
| Tannin + formaldehyde (TF) | Hydrogels prepared at 85 °C for 120 h; Acetic acid or NaOH were used to change the initial pH of the solution (4.3); pH = 3.3−7.3; Freeze-drying; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min |
Carbon cryogels; SBET = 399–1420 m2/g; Porosity = 94–96% Carbon cryogels presented high pore volumes, mostly distributed in macro- and micropores sizes. These materials also presented very low bulk densities (0.078–0.101 g/cm3), mostly composed by macropores (0.13–0.55 cm3/g). Materials prepared at pH higher than 6 presented higher SBET (1228–1420 m2/g) and higher micropore volumes. The electrochemical performances of those materials were tested, and the main results are presented in Table 3. |
[126] |
| Tannin + pluronic + formaldehyde (TPF) | Hydrogels prepared at 85 °C for 120 h; pH from 2 to 10; Subcritical drying in two steps: air and 80 °C; Mass ratio of T/P of 0.5–2; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min |
Carbon xerogels; SBET =5–877 m2/g; Porosity = 46%–86% The use of pluronic allowed the synthesis of the first carbon tannin based xerogels presenting density values in a range of 0.28–0.33 g/cm3, which are comparable to those of carbon aerogels derived from expensive precursors. Those densities are related to samples prepared at pH lower than 7 and low amounts of pluronic (Mass ratio of T/P of 2). Narrow bimodal macro-microporous or meso-microporous xerogels were produced as a function of pH and surfactant. For lower pluronic concentrations, the micropore size distribution remained centered at 0.5 nm, while the macroporosity was shifted as a function of both pluronic and pH. By increasing the amount of pluronic and keeping the same pH, the porosity changed from a macro-microporous structure to a meso-microporous structure. The final structure was highly porous with narrow non-ordered porosity. |
[98] |
| Tannin (T) (autocondensation) | Tannin was added to solutions of Na2SiO3 (35 wt.%); Mass ratio of SiO2/T of 0.3–1.04; NaOH (2 mol/L) or HF (40 wt. %) were used as etching agents; Curing and ageing were performed at ambient temperature for 3 h; Supercritical drying in CO2; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min |
Carbon aerogels; SBET NaOH = 451–709 m2/g; SBET HF= 542–783 m2/g; Porosity = 67%–82% Porous carbon gels were prepared with no formaldehyde addition. Na2SiO3 was used as catalyst for auto-condensation and as template for porosity formation. The final porous properties were controlled by silica/tannin ratio, while NaOH or HF were used as etching agents. XPS and Raman analyses showed the non-ordered aromatic structure of tannin-based carbon aerogels. Carbon aerogels washed with HF solutions presented better porosity (71%–82%) compared to NaOH (67%–75%). Samples prepared at low SiO2/T mass ratio (0.30–0.75) had no significant differences. However, the highest SiO2/T mass ratio (1.04) presented the lowest density, and consequently the highest porosity. By increasing the quantity of silica in the reactional medium, there was a decrease in the microporosity associated to SBET, whereas the highest values were found for SiO2/T = 0.45. Mesopores were better developed at SiO2/T mass fraction of 0.75 (1.71 cm3/g for samples etched with HF). |
[127] |
| Tannin + sodium dodecyl sulfate + formaldehyde (TSDSF) | Hydrogels prepared at 85 °C for 72 h; NaOH and sodium dodecyl sulfate (SDS) were used as catalyst and surfactant, respectively; Initial pH of 5.5, SDS amount of 5–20 wt.% and T/F mass ratio of 0.6–2.6; MFTS of 25 wt.%; Supercritical drying in CO2; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min |
Carbon xerogels; Porosity = 25%–78% There was an optimal amount of surfactant (10 wt.%) for producing highly porous carbon xerogels (density of 0.34 g/cm3 and porosity of 78%). However, when using 10 wt.% of surfactant and increasing the T/F mass ratio, the pore volumes increased, and the average pore size decreased. Also, the concentration of formaldehyde was important for attaining materials with greater porosity, since an ideal proportion of crosslinker promotes the creation of links between surrounding clusters. |
[97] |
| Tannin + formaldehyde (TF) | Hydrogels prepared at 85 °C for 120 h; pH of 2, 5 and 8; Activation with NaOH or KOH; Mass ratio of NaOH/gel of 0.045, 0.181 and 0.724 (on a dry basis); Mass ratio of KOH/gel of 0.063, 0.253 and 1.013 (on a dry basis); Activation: 750 °C for 1 h; Heating rate of 5 °C/min |
Activated carbon xerogels (ACX); SBET = 50–1810 m2/g The exchange step was performed with alkali solutions at different concentrations. Higher concentrations of alkali (NaOH or KOH) induced the development of surface area. In addition, the microstructure of ACX changed from nodular to microcellular carbon foam and, the effect of the pH was not meaningful in these different structures. A smaller dispersion of particle sizes and high surface areas related to micropores volumes were obtained using smaller mass ratio of alkalis (0.045–1.013), compared to usual values in the literature (>1). Probably, the soft structure of gels allowed the alkali solution to interpenetrate in its three-dimensional structure, causing greater alkali diffusion and favoring the formation of higher porosity. |
[118] |
2.4. Hydrogels Formulations under Hydrothermal Conditions
| Precursors | Conditions | Main Findings | Ref. |
|---|---|---|---|
| Hydrogels Prepared Under Hydrothermal Conditions Organic and Carbon Hydrothermal Tannin Gels |
|||
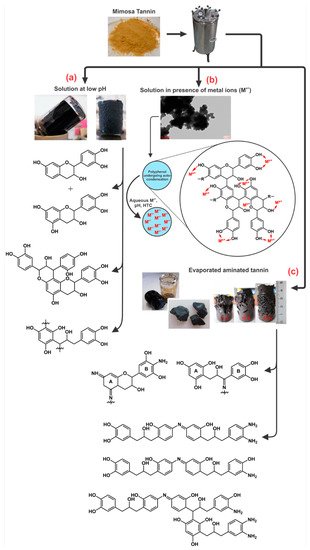
| Evaporated aminated tannin (EAT) | Hydrothermal gel: Amination at room temperature followed by evaporation and HTC of the solid material in water at 180 °C for 24 h; Subcritical drying in two steps: air and 80 °C; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. |
Xerogel: SBET = 32 m2/g This amination process under HTC conditions lead to a gel without any crosslinker agent. The mechanism was based on autocondensation and partly dehydrated tannin mainly through the heterocycle opening. Materials also had high percentage of nitrogen (up to 3.4%). Carbon xerogel: SBET = 500 m2/g The synthesized carbon gel had a porous structure comprised of a mixture of wider microporosity and mesoporosity (Isotherms Type I and IV). Materials had high percentage of nitrogen (up to 2.9%) and a micropore/total pore volume ratio of 72%. XPS analysis showed several forms of nitrogen such as pyridinic (N-6) and neutral amines in the hydrothermal xerogel, whereas pyridic (N-6), pyrrolic (N-5) and oxidized (N-X) (stable forms of nitrogen) were found in its carbon form. |
[105] |
| Evaporated aminated tannin (EAT) | Hydrothermal gel: Amination at room temperature followed by evaporation and HTC of the solid material in water at 180–220 °C for 24 h; Subcritical drying in two steps: air and 80 °C; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. |
Xerogel: SBET = 64−102 m2/g A monolithic gel was obtained under HTC at temperatures from 180 to 220 °C. Not many differences in relation to the physicochemical properties were observed in this range of temperature. These gels were nitrogen rich materials (up to 4.3%). Carbon xerogel: SBET = 311−552 m2/g Higher HTC temperatures lead to lower surface areas (~ 300 m2/g) due to the formation of bigger and less porous spherical nodules. After carbonization, materials still had a high percentage of nitrogen (up to 2.9%). N-doped materials were successfully synthesized for electrochemical devices. These results were presented and discussed in Table 3. |
[109] |
| Evaporated aminated tannin (EAT) | Hydrothermal gel: Amination at room temperature followed by evaporation and HTC of the solid material at mass fractions of 11, 18 and 27% in water at 180 °C for 24 h. The final gels were first soaked in ethanol for 3 days, and then subcritically (air and 80 °C), supercritically (CO2), and freeze-dried; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. |
Xerogel, aerogel and cryogel: SBET = 102−295 m2/g For the most diluted gels (11 wt.%), the porosity improved in this sequence: xerogel, cryogel and aerogel. For the least diluted gels, similar surface areas were obtained at any kind of drying method. The mechanism of gel formation was described in Section 2.4. Carbon xerogel, aerogel and cryogel: SBET = 496−860 m2/g Unlike organic gels, carbon gels prepared with the highest mass fraction (27 wt.%) had the highest surface area, up to 900 m2/g. It was then noticed that the most porous organic gels do not lead to carbon gels with the highest surface areas. |
[111] |
| Tannin (T) | Hydrothermal gel: Tannin aqueous solution under HTC conditions at 180 °C for 24 h; pTSA (para-toluenosulfonic acid) was used to change the pH from 4.2 to 1; Subcritical drying in two steps: air and 80 °C; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. |
Xerogel: SBET = 1.25 m2/g Monolith material was obtained by reducing pH, resulting in a positive impact on the hydrochar yield. However, a very low surface area was obtained. Carbon xerogel: SBET = 796 m2/g Monolithic materials having spherical nodules at different diameters were obtained: 2.9 µm at pH 2; and 4.6 µm at pH 4.2. This trend was different from phenolic gels prepared with tannin-formaldehyde. The surface area of 796 m2/g was attained for the material prepared at lowest pH. Its higher microsphere nodule diameter might have promoted the gasification of low molecular weight gases, which enhanced the development of their surface area. |
[106] |
| Evaporated aminated (pine) tannin (EAT) | Hydrothermal gel: Amination at room temperature followed by evaporation and HTC of the solid material in water at 180 °C for 24 h; Subcritical drying in two steps: air and 80 °C; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. |
Xerogel: Compacted monoliths having homogeneous spherical particles typical of gels. Carbon xerogel: SBET = 485 m2/g Micro-mesoporous carbon gels exhibited high surface area with high nitrogen and oxygen functionalized groups, which were applied as electrodes for electrochemical devices. These results were described in Table 3. |
[128] |
| Bayberry tannin and graphene oxide (GO) | Hydrothermal gel: A mixture of graphene oxide and tannin at different mass ratios (1:0–1.5); Sonication for 30 min and HTC at 120 °C for 12 h.; Gels were soaked in HNO3 solution for 4 days; Freeze-drying. |
Hydrothermal cryogel: SBET = 75 m2/g The monolith had a three-dimensional structure, consisting of crosslinked GO sheets, GO sheets, and tannin. The gels showed a type III isotherm characteristic of mesoporous materials, having surface area up to 75 m2/g. Its application as adsorbent to remove Sr2+ from water is described in Table 3 |
[112] |
2.5. Thermal Treatment of Organic Gels

| Application | Type of Gel | Testing Conditions | Main Findings | References |
|---|---|---|---|---|
| Organic tannin gels | ||||
| Thermal insulator | Aerogel | Thermal conductivity of soy-tannin-formaldehyde in a cylindrical shape was performed at room temperature | Tannin gel conductivity is higher than that of air (0.033 W/mK compared to 0.026 W/mK). The presence of narrow mesopores and fewer macropores would be required to improve their performance as thermal insulators. | [54] |
| Thermal insulator | Aerogel | Thermal conductivity of tannin-formaldehyde in a cylindrical shape was performed at room temperature | Tannin gel conductivity is close to that of air (0.027 W/mK). Low thermal conductivity was reported for gels with low density and very narrow pores. | [60] |
| Adsorbent for water treatment | ||||
| Metal Pb2+ | Tannin-gel | Synthetic effluent 1000 mg/L, batch tests; ratio metal: adsorbent 0.1 g: 100 mL; Equilibrium time 5 h, pH = 1−7, T = 20 °C | Tannin-gel behavior as ionic exchanger: two Na+ ions exchanged by one Pb2+ ion. Pb removal efficiency increased from 58 to 115 mg Pb/g with increased pH from 3 to 4.2, respectively. | [84] |
| Metal Cr6+ | Tannin-gel | Synthetic effluent 1000 mg/L, batch tests; ratio metal: adsorbent 2.5 g:100 mL; Equilibrium time 30 min, pH = 0.85–5, T = 30 °C | Adsorption mechanism consists of four steps: 1) Esterification of chromate with tannin molecules; 2) Reduction of Cr6+ into Cr3+; 3) Formation of carboxyl groups by tannin oxidation; and 4) Ion exchange of Cr3+ with carboxyl and hydroxyl groups. Maximum adsorption capacity: 287 mgCr/g at pH 2. |
[83] |
| Metal Cr6+ | Tannin-gel | Synthetic effluent 500–5000 mg/L, batch tests; ratio metal: adsorbent 0.2 g:100 mL; Equilibrium time 8 h, pH = 1–12, T = 25–45 °C | Cr adsorption reached the maximum value of 488 mg/g at pH 1 (25 °C). The mechanism of Cr adsorption is based on: 1) Cr(IV) adsorption by phenolic groups through chromate esterification with tannin-gel surface; 2) Cr(VI) reduction to Cr(III); 3) Carboxylate group formation due to tannin-gel oxidation; 4) Cr(III) retention on tannin-gel surface; and finally 5) Cr adsorption through hydroxyl and carboxyl groups. | [138] |
| Metal Cu2+ | Tannin-gel | Synthetic effluent 10−150 mg/L, batch tests; ratio metal: adsorbent 0.1 g:100 mL; Equilibrium time 3 h, pH = 2−5, T = 25 °C | Adsorption mechanism is a result of ion exchange or complexation between Cu2+ ions and phenolic groups present on tannin-gel surface. Adsorption decreases at lower pH due to ion exchange equilibrium. Maximum adsorption capacity: 44 mgCu/g at pH 5. | [87] |
| Metal Zn2+ | Tannin-gel | Synthetic effluent, batch tests; Equilibrium time 2 weeks, pH = 7, T = 20 °C | Tannin-gel made from lab-extracted Pine tannin presented the best performance for Zn removal. Maximum adsorption capacity: 65 mgZn/g |
[88] |
| Metal Ni2+ | Tannin-gel | Synthetic and real effluent (synthetic and river water) 50−200 mg/L, batch tests; ratio metal: adsorbent 0.25−1 g: 100 mL; Equilibrium time 35 min, pH = 2–7, T = 23 °C | The adsorption of Ni ions took place in a homogeneous tannin gel surface (monolayer adsorption). Maximum adsorption capacity: 250 mgNi/g at pH 5 |
[139] |
| Metal Sr2+ | Hydrothermal cryogel | Synthetic effluent 10−150 mg/L, batch tests; ratio metal: adsorbent 0.02 g:100 mL; Equilibrium time 10 h, pH = 9, T = 25 °C | Graphene oxide-tannin gel prepared under HTC conditions showed excellent adsorption performance for the removal of Sr2+ (68 mgSr/g). Surface chemical analysis showed that Sr2+ was largely dependent on oxygen functional groups, pH, salinity and ionic strength. | [112] |
| Rare metal V | Tannin-gel | Synthetic effluent 0.2 mM, batch tests; ratio metal: adsorbent 0.02 g:100 mL; Equilibrium time 1 h, pH = 1–8, T = 30 °C | V was efficiently adsorbed from different solutions: VOCl2 and NH4VO3. Stable compounds were formed between VO2+ (acid character) and catechol and pyrogallol (alkali behavior). V adsorption from NH4VO3 was based on adsorption of H3VO4 (pH = 3.75) and reduction of VO2+ to VO3- at pH 6. | [140] |
| Rare metal Au | Tannin-gel | Synthetic effluent 10 mg/L, batch tests; ratio metal: adsorbent 0.01 g:100 mL; pH = 2−3.8, T = 20 °C | Adsorption of Au took place through the reduction of trivalent Au ions into metallic Au as well as oxidation of hydroxyl groups present in tannin-gel to carbonyl groups. Maximum adsorption capacity: 8000 mgAu/g |
[85] |
| Rare metal Au | Tannin-gel | Synthetic effluent 0.1 mM, column tests; Flow bed at 150 and 300 mL/h; pH = 2–6 | 98.5% of Au from HAuCl4 was adsorbed at pH 6. The mechanism of Au adsorption was based on: 1) Ligand exchange: AuCl4- with hydroxylphenyl groups present in the tannin-gel; 2) Reduction of Au(III) to Au(0); and 3) Adsorption of Au(0). | [141] |
| Rare metal Pd(II) | Tannin-gel | Synthetic effluent 10 mg/L, batch tests; ratio metal: adsorbent 0.04 g:100 mL; pH = 1.3−2.5, T = 25 °C | Adsorption of Pd(II) was based on the inner sphere redox reaction: Pd(II) ions were adsorbed as metallic Pd; hydroxyl groups were oxidized; and a ligand-substitute Pd(II) tannin inner sphere complex was formed. | [86] |
| Rare metal Pd(II) | Aminated tannin-gel freeze-dried | Synthetic effluent 100 mg/L, batch tests; ratio metal: adsorbent 0.1 g:100 mL; Acidic medium, T = 25 °C | Adsorption of Pd(II) was due to metal ions complexation and/or electrostatic interaction. Also, acidic metal ions had high affinity towards amine basic groups. Maximum adsorption capacity: 80 mgPd/g. |
[142] |
| Rare metals Pd and Pt | Aminated tannin-gel freeze-dried | Synthetic effluent 0.001 M, batch tests, single and multiple metal solutions; ratio metal: adsorbent 0.1 g:100 mL; Equilibrium time 20 h, pH = 0–5, T = 25 °C | The adsorption of Pd and Pt on tannin-gel surface increased with increasing pH and temperature, and with decreasing chloride ion concentration. The amino groups presented in tannin-gel formed stable complex with metal ions but the adsorbability of Pd(II) was much higher than Pt(IV). Interesting that aminated tannin gel adsorbed mostly Pd(II) from mixed solutions even though it had good adsorbability for Pt(IV) from single metal solution | [143] |
| Rare metals Au, Pd, Pt and Rh | Tannin-gel | Synthetic effluent 1 mmol/L, batch tests, single and multiple solutions; ratio metal: adsorbent 0.1 g:100 mL; pH = 1, T = 25 °C | The predominant species of each metal were adsorbed by controlling the pH (equal to 1) as well as the redox potential differences between metals and tannin-gel. Au was selectively adsorbed and reduced because its redox potential was higher than that of tannin-gel. However, the other precious metals had much lower redox potential than that of tannin-gel. | [144] |
| Metals: Au(III), Pd(II), Pt(IV), Cu(II), Fe(III), Ni(II), Zn(II) | Tannin-gel | Synthetic and real effluent, batch and column tests, single and multiple metal solutions; ratio metal: adsorbent 0.1–2 g:100 mL; Flow bed at 5 mL/h; Equilibrium time 12 h, acidic pH, T = 30 °C | Rare metals were efficiently adsorbed through both batch and column tests. Tannin-gel selectively adsorbed Au(III), Pd(II) and Pt(IV) over other metals: Cu(II), Fe(III), Ni(II) and Zn(II). The mechanism of adsorption of precious metals was the combination of ion exchange, electrostatic interaction and coordination with thiocarbonyl group. Au(III) was reduced to elemental Au through abundant polyphenolic groups on tannin molecule. Tannin-gel was regenerated under acid solution up to five times. | [145] |
| Boron (B) | Aminated tannin-gel freeze-dried | Synthetic effluent 200 mg/L, batch tests; ratio contaminant: adsorbent 0.5 g:100 mL; Equilibrium time 20 h, pH = 8.8, T = 30 °C | Aminated and non-aminated gels efficiently adsorbed B at pH > 7. The adsorption of B took place through the chelate formation of tetrahydroxyborate ion and the hydroxyl and amino groups presented in tannin-gels. The adsorption capacity of the aminated cryogel was higher than that of non-aminated one due to the stable bonds between boron and nitrogen from amino groups. | [146] |
| Phosphate (P) | Tannin-gel freeze-dried | Synthetic effluent 100 mg/L, batch tests; ratio contaminant: adsorbent 0.5 g:100 mL; pH = 2−12, T = 25 °C | The gel impregnated with iron and oxidized with nitric acid showed adsorption selectivity for phosphate. The adsorption process was independent of the pH (from 3 to 12). Maximum adsorption capacity: 31 mgP/gFe |
[147] |
| Organic MB | Tannin-gel | Synthetic effluent, batch tests; Equilibrium time 2 weeks, pH = 7, T = 20 °C | Tannin-gel made from lab-extracted Pine tannin presented the best performance for MB removal. Maximum adsorption capacity: 432 mgMB/g |
[88] |
| Organic MB | Tannin-gel | Synthetic effluent 1000 mg/L, batch tests; Equilibrium time 15 days; pH = 4−10, T = 20 °C | Adsorption of MB was improved by increasing pH, probably because the dye appeared with a higher cationic degree and thus, it enhanced electrostatic interactions. Maximum adsorption capacity: 483 mgMB/g |
[148] |
| Organic BR | Tannin-gel | Synthetic effluent 40 mg/L, batch tests; ratio contaminant: adsorbent 0.04 g:100 mL; Equilibrium time 1.5 h, pH = 2–8, T = 28 °C | Good adsorption of BR due to the presence of functional groups on tannin-gel structure: phenolic, carboxylic, alcoholic, ether and aromatic rings. Maximum adsorption capacity: 45 mgBR/g | [149] |
| Organic CTAB | Tannin-gel | Synthetic effluent, batch tests; Equilibrium time 2 weeks, pH = 7, T = 20 °C | Tannin-gel made from lab-extracted Pine tannin presented the best performance for CTAB removal. Maximum adsorption capacity: 773 mgCTAB/g |
[88] |
| Benzene and toluene | Tannin-gel | Synthetic effluents 1% sol., batch tests; Equilibrium time 1 h; ratio contaminant: adsorbent 0.1 g:100 mL; pH = 2–8.6, T = 60 °C | The removal of toluene was more effective than benzene probably because of the interactions between the methyl groups on toluene and the OH groups on tannin gel. Results show up to 99% removal of toluene and benzene after 30 min batch tests. | [150] |
| Carbon tannin gels | ||||
| Thermal insulator | Carbon xerogel | Thermal conductivity of tannin-formaldehyde-surfactant in a cylindrical shape was performed at room temperature | Carbon tannin gel conductivity is higher than that of air (0.039 W/mK compared to 0.026 W/mK). The presence of narrow mesopores would be required to improve its performance as thermal insulator. | [97] |
| Electrochemistry | ||||
| Supercapacitor | Carbon cryogel | Supercapacitor device based on a three-electrode cell configuration with an aqueous acid electrolyte (4 M H2SO4) | Carbon cryogels prepared at pH higher than 6 had low density and high surface areas (up to 1200 m2/g). Thus, such materials as electrodes for supercapacitor reached capacitances as high as 100 F/g (scan rate 2 mV/s). In addition to mesopores, ultra- and supermicropores played an important role on their performance as electrodes for supercapacitor. | [126] |
| Supercapacitor | Hydrothermal carbon aero-, cryo- and xerogel | Supercapacitor device based on a three-electrode cell configuration with an aqueous acid electrolyte (4 M H2SO4) | Carbon aerogel, cryogel, and xerogel prepared from HTC of evaporated aminated tannin (MFTS of 27 wt.%) reached surfaces areas of 860, 754, and 585 m2/g and specific capacitances of 362, 387, and 330 F/g (scan rate 2 mV/s), respectively. The presence of nitrogen (2−3 wt.%) and oxygen (17−18 wt.%) functional groups played an important role on their performance for electrical energy storage, especially through pseudo-capacitance. However, mesostructuration within 3−13 nm should be created to improve the capacitance reduction at a higher scan rate. | [111] |
| Supercapacitor | Hydrothermal carbon xerogel | Supercapacitor device based on a three-electrode cell configuration with an aqueous acid electrolyte (1 M H2SO4) | Hydrothermal carbon xerogel made from evaporated aminated Pine tannin reached a surface area and a specific capacitance of 485 m2/g and 253 F/g (scan rate 0.5 mV/s), respectively. The material presented high concentration of nitrogen and oxygen functional groups (6 mmol/g) that played an important role on their performance as electrodes for a supercapacitor. | [128] |
2.6. Applications of Organic and Carbon Tannin Gels
This entry is adapted from the peer-reviewed paper 10.3390/biom9100587
