The detection of glucose is crucial in the management of diabetes and other medical conditions but also crucial in a wide range of industries such as food and beverages. The development of glucose sensors in the past century has allowed diabetic patients to effectively manage their disease and has saved lives. First-generation glucose sensors have considerable limitations in sensitivity and selectivity which has spurred the development of more advanced approaches for both the medical and industrial sectors.
- enzymatic
- non-enzymatic
- glucose sensor
- glucose oxidation
- electrochemical sensor
1. Introduction
The increased sugar consumption in people’s diet is related to many chronic health problems including cardiovascular diseases (including heart failure, stroke or heart attack), type 2 diabetes, sleep apnea, metabolic syndrome, and obesity [1][2]. In 2019, diabetes affected 463 million people worldwide, was responsible for 1.5 million deaths, and the number of diabetic patients is expected to increase to 700 million by 2045 [3]. Moreover, diabetes is associated with other pathologies such as the risk of blindness, kidney failure, nerve damage, and heart problems [4]. Therefore, diabetic patients need to accurately determine their glucose blood level not just at the diagnosis stage but in all stages of treatment and disease management, and the use of non-invasive and rapid glucose level testing methods is critical [5][6][7][8].
In the last decade, the demand for glucose detection and monitoring systems significantly increased. This is reflected in the increased number of publications related to glucose sensors, illustrated in Figure 1. Glucose sensors comprise optical and electrochemical sensors. Optical glucose biosensors, encompassing different optical methods such as fluorescence, absorptiometry, and surface plasmon resonance (SPR)
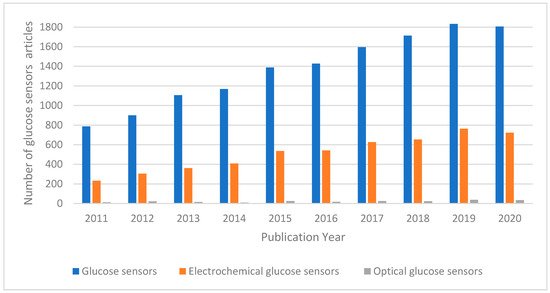
Electrochemical sensors, mainly based on amperometric methods, represent the most relevant group of glucose biosensors and comprise enzymatic and non-enzymatic sensors. Noble metals and their composites have been used specifically as the electrode materials for non-enzymatic sensors due to their high electrocatalytic activity, and high sensitivity to the electrooxidation of glucose [9][10][11][12]. The major problem faced by non-enzymatic glucose sensors is the absorption of glucose oxidation intermediates (e.g., CO) or solution active species (e.g., Cl−) which can lead to blockage of electrode activity for direct glucose electro-oxidation [13]. Furthermore, non-enzymatic amperometric glucose sensors suffer from a lower selectivity compared to enzymatic amperometric glucose biosensors due to the difficulty faced by the electrocatalytic materials to specifically catalyse glucose oxidation.
The principle behind enzymatic amperometric glucose sensors was proposed by Clark and Lyon in a patent describing the use of enzymes for converting electroinactive substrates into electroactive products [14]. Clark [15] also designed the first enzymatic amperometric glucose sensor by immobilising glucose oxidase (GOx) on a platinum (Pt) electrode. Since these preliminary studies, GOx has been extensively investigated and used for glucose biosensors due to its low cost, high bioactivity, selectivity, and stability [16]. Glucose dehydrogenase (GDH) is also used for blood glucose test strips [17][18][19][20].
Glucose sensing has a significant scientific, clinical, and industrial relevance and significant progress has been made recently, particularly related to non-invasive methods to monitor blood [21][22][23][24].
2. Three Generations of Enzymatic Glucose Sensors
The concept of a glucose enzyme electrode, as proposed by Clark and Lyon [14], monitors the oxygen consumption according to the following enzyme-catalysed reaction:(1)glucose+oxygen→GOxgluconic acid+hydrogen peroxid
The main problem faced by this sensor was the interference from background oxygen during the reaction. To solve this problem, Updike and Hicks [25] developed a system based on two oxygen working electrodes, measuring the current differential, hence, removing the noise created by the background oxygen. Similarly, Guilbault and Lubrano [26] developed an enzymatic amperometric glucose biosensor by monitoring the released hydrogen peroxide (H2O2) as follows:(2)H2O2→O2 +2H++2e−
The catalytic reaction of GOx-based glucose biosensors involved the reduction of the enzyme’s flavin group (GOx(FAD)) to the reduced form (GOx(FADH2)) [27]:(3)GOx(FAD)+glucose→GOx(FADH2)+gluconic acid
The reduction is then counteracted using an electron acceptor and oxidation mediator, (Medox) to reoxidise the enzyme and regenerate the oxidised form (GOx(FAD)) [27]:(4)GOx(FADH2)+Medox→GOx(FAD)+Medred
The regeneration of the enzyme is important to guarantee the enzymatic cycle, otherwise the enzyme will be reduced and cannot be reused, ending the sensing process.
According to the type of oxidation mediator, it is possible to identify three generations of glucose biosensors as shown in Figure 2. The first-generation of sensors use O2as a physiological mediator, the second-generation uses an artificial (synthetic) electron acceptor, while the third-generation uses an electrode for direct electrical communication without requiring any mediators.
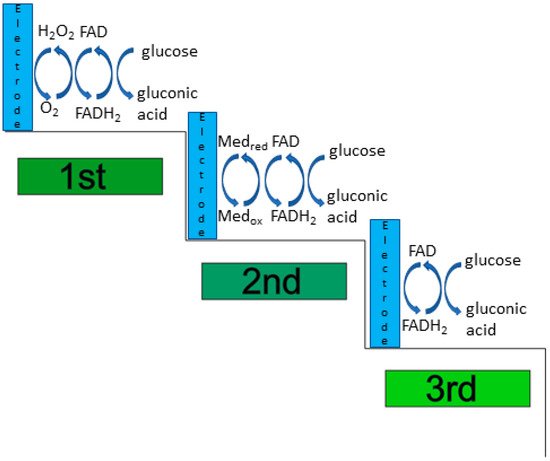
The first generation of enzymatic glucose biosensors relied on oxygen as the oxidation mediator to regenerate GOx(FAD), thus detecting glucose by monitoring the oxygen consumption, or the generation of H2O2during the enzymatic reaction [28]. The anodic oxidation and cathodic reduction of H2O2were used to monitor the enzymatic generation process [29]. Moreover, the anodic oxidation of H2O2enhances the ability to regenerate/replenish the oxygen, improving the enzymatic cycle [29]. The first-generation of enzymatic glucose biosensors were stable, simple, and easily used in miniaturised applications [30].
At a high potential level, some coexisting species such as ascorbic acid and uric acids are electroactive, reducing the selectivity and accuracy of the biosensor [31][32]. This problem was minimised by using a permselective membrane, reducing the access of the interferent to the surface of the biosensor transducer [33][34][35]. (Os) complexes were used based on their transport properties, pore size, charge, or polarity [36][37][38][39]. Similarly, Wang and Wu [40], developed a glucose biosensor with high selectivity by dispersing rhodium particles in an Nf film.
The aim was to develop a highly selective and low potential glucose biosensing, due to the PB having high catalytic activity and selectivity for the reduction of H2O2. [41] produced a highly sensitive imprinted electrochemical sensor based on double amplification using an inorganic PB catalytic polymer and GOx [41]. Moreover, a wide range of nanomaterials including carbon nanotubes (CNTs) , Pt nanoparticles [42], and composite nanomaterials [43][44] were successfully used to improve selectivity due to their high catalytic effect.
Another important limitation of the first generation of glucose biosensors, based on the use of oxygen as Medox, was related to oxygen dependence [25][45]. These sensors were prone to errors, due to oxygen tension fluctuation and the stoichiometric limitation of oxygen, usually referred to “oxygen deficit” (the normal oxygen concentration is an order of magnitude lower than the physiological level of glucose) [46]. Other approaches included the use of an oxygen-rich carbon paste enzyme electrode [35][47][48] or an air diffusion biocathode that used oxygen directly from the air [49].
The second generation of enzymatic glucose biosensors relied on the use of an artificial Medoxto mediate the GOx cycle instead of depending on oxygen as a mediator to transport electrons to and from the enzyme active site [50]. The artificial Medoxcan be an immobilised mediator directly attached to the enzyme or entrapped in an enzyme film [51][52], a solution-state mediator able to diffuse in and out of the enzyme active site [46], or a redox-conducting polymer able to transport its electrons to and from the enzyme active site [18][53][54]. Suitable mediators for GOx include conducting organic salts (particularly tetrathiafulvalene-tetracyanoquinodimethane, TTF-TCNQ), ferrocene, quinone compounds, ferricyanide, transition-metal complexes, phenothiazine, and phenoxazine compounds [30][55][56][57][58][59].
The catalytic process consisted of three steps: (1) the reduction of the GOx(FAD) to GOx(FADH2) due to the electron transfer from the glucose to the FAD reaction centres of GOx; (2) electrons transfer from the FADH2centres to the artificial mediator (Medox), hence reducing it from Medoxto Medred; and (3) the transport of electrons through the artificial mediator to the electrode [46]. A current signal is produced due to the oxidation of Medredand used for glucose measurement, which requires an efficient interaction between the enzymes and the mediators to guarantee the effective transportation of the electrons between the redox active centres and the electrode [51].
Several approaches have been proposed to tailor the mediators in the electrode-supported enzyme films, including using Os complex as a mediator, non-covalent functionalisation of multiwalled carbon nanotubes (MWCNTs), GOx and binding proteins, and stabilising artificial mediators [60][61][62]. [63], designed a bienzymatic glucose biosensor based on the non-covalent functionalisation of MWCNTs with GOx and avidin (to allow the specific anchoring of biotinylated horseradish peroxidase (b-HRP)). [46] designed a reagentless biosensor with free diffusing mediators by covalently bonding the GOx to the surface of the biosensor followed by exposing it to a water-organic mixture containing a high content of organic solvent [46]. In the case of immobilised mediator-based biosensors, it is important to immobilise the artificial mediator near both the enzyme’s redox centre and the electrode surface to ensure high electron-exchange efficiency.
The third generation of enzymatic glucose biosensors relies on direct energy transmission (DET), which depends on the distance between the enzyme’s redox centre and the electrode surface [64][65]. The reassembling of apo-proteins on cofactor modified enzymes and the reassembling of apo-enzymes on cofactor Au nanoparticles (AuNPs) are widely used strategies to align redox enzymes on the electrodes [66][67][68][69][70]. These methods are effective in the process of electrically wiring the redox enzyme to the electrode surface but are complex processes which limit their usage. [71] achieved direct energy transfer between GOx and electrode via a site-specific modification of GOx to display a free thiol group near the active site, hence facilitating site-specific attachment of maleimide modified AuNPs to the enzyme.
This third generation of glucose sensors produced better results than both the first and second generation, but still present restrictions stemming from their dependency on the enzyme’s activity which can be influenced by external environmental factors such as temperature, pH, and humidity [64][72][73]. Moreover, the biosensor performance also depends on the enzymatic layer thickness with high layer thickness resulting in signal dampening or loss [74][75].
Despite all these developments, the different generations of biosensors present several limitations not yet fully addressed, which has led to the development of non-enzymatic glucose detection systems. These non-enzymatic glucose sensors, sometimes referred to as the fourth generation of glucose sensors, rely on the concept of oxidising glucose directly on the electrode surface.
3. Recent Developments in Enzymatic Glucose Biosensors
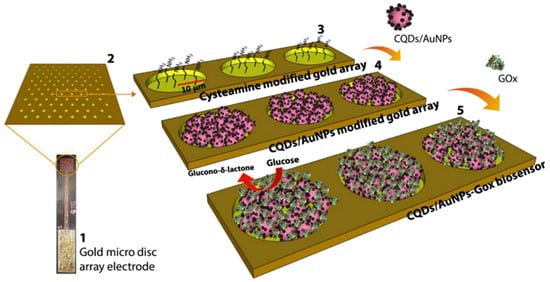
Advances in the field of nanomaterials have led to the development of enzymatic biosensors incorporating nanomaterials (e.g., noble and transition metal nanoparticles, CNTs, graphene, and nanostructured metal oxides) to amplify the electron transfer rate, improving the biosensor performance in terms of selectivity and sensitivity [76]. GOx was then covalently immobilised to the Au-SiO2NP followed by drop casting of the SiO2NP/GOx solution on the surface of a glass electrode. Buk and Pemble [77] prepared a glucose biosensor using a micro disk array electrode, modified with carbon quantum dots (CQDs)-AuNPs as a matrix for GOx (Figure 3). Finally, glutaraldehyde was used to immobilise GOx, producing a micro disk array with a sensitivity of 626.06 μAmM−1cm−2and a wide linear range from 0.16 to 4.32 mM [77].
MWCNTs were recently investigated to produce immobilisation matrices for GOx due to their high stability and ability for direct electron transfer [78][79][80]. [78] developed a bio-nanohybrid material by dispersing functionalised MWCNTs (fMWCNTs) in a Nf film dopped with PPy. [79] used cobalt (II) sulphide nanoparticles (CoSNPs) to coat MWCNTs through an in situ hydrothermal method, obtaining a CoS-MWCNTs composite used as a matrix for GOx immobilisation. [80] developed a functional nanocomposite by depositing manganese dioxide (MnO2) on the surface of MWCNTs via an in situ hydrothermal method.
Similarly, graphene has been used to produce enzymatic glucose biosensors. [81] using MnO2nanoparticles to decorate graphene nanoribbons (GNR) followed by surface modification using a drop coating method with GOx and Nf. The composite solution was then drop cast on the screen printed carbon electrode (SPCE) producing a MnO2-GNR/SPCE electrode, followed by the addition of GOx and Nf, which resulted in an enzymatic glucose biosensor with a sensitivity of 56.32 μA [82] investigated the use of reduced graphene oxide (rGO) to increase the sensitivity and selectivity of a zinc oxide (ZnO) nanorod based biosensor.
Recently, Hossain and Slaughter [83] proposed a hybrid glucose biosensor with high sensitivity and selectivity using both MWCNTs and graphene. Chemically derived graphene and MWCNTs functionalised with carboxylic groups were synthesised using a one-step solvothermal technique to produce a suspension containing both materials. The fabricated hybrid biosensor exhibited sensitivity of 26.5 μA mM−1cm−2and linear detection range from 0.5 to 13.5 mM [83].
High selectivity is a key requirement for glucose sensing applications. One approach to improve selectivity consists of using a red blood cell membrane (RBCM) as a diffusion barrier on the surface of the enzymatic glucose biosensor to eliminate any interfering molecules from reaching the surface [84]. RBCM collected from red blood cells was used to coat the outer surface of the coated SPGE. The RBCM coated enzymatic glucose biosensor was then tested, showing lower limit of detection than the uncoated biosensor demonstrating that the RBCM increases the biosensor selectivity and its performance [84].
Conductive polymers (CP), prepared mostly by incorporating conductive nanoparticles within a polymer matrix, can be used for enzyme immobilisation due to their unique properties such as high electron affinity, electrical conductivity, redox activity, stability, and low cost [85][86]. [87] fabricated an enzymatic glucose biosensor using a novel electrochromic conductive polymer, poly(2,5-di(furan-2-yl)thiazolo[5,4-d]thiazole) (PTTzFr), to immobilise GOx. [88] developed a ratiometric enzymatic glucose biosensor using schiff base polymers (SBPs) due to their stability, biocompatibility, and good mechanical and catalytic properties. The electrodes were coated with chitosan as an immobilisation matrix for GOx allowing the covalent bonding of the GOx to its surface via the active amine (NH) side group, improving stability and preserving the biocatalytic functions of the enzyme.
Enzymatic glucose biosensors for blood glucose monitoring led to the development of patient friendly devices, enabling continuous and real-time glucose monitoring. This was a three-layered sensor based on a glucose sensing layer, a glucose mass transport restricting layer, and an outer biocompatible layer. Below the glucose sensing layer, a flexible gold wire electrode was used. The ETC depends on the HA penetration into the interstitial fluid (ISF) (anode channel), intravascular blood glucose refiltration from vessels, and glucose reverse iontophoresis to the skin surface (cathode channel).
4. Recent Developments in Non-Enzymatic Glucose Sensors
Non-enzymatic glucose sensing is a cheap and rapid technique that relies on the direct electrochemistry of glucose (oxidation or reduction) However, direct glucose oxidation on noble metal electrodes suffer from three major limitations [89][90][91][92][93]: (1) restricted glucose sensitivity which can be attributed to the slow glucose electro oxidative kinetics on conventional electrodes; (2) low selectivity as several sugars can be oxidised in the same potential range as glucose; and (3) reduced electrode activity due to ion contamination, mainly chloride ions (Cl−). The sensitivity and selectivity limitation can be countered by increasing the surface area of the electrode allowing more glucose to be in direct contact with the electrode’s surface. Particularly relevant are noble metals such as Pt, nickel (Ni), Ag, zinc, and Au, which are highly utilised to develop novel non-enzymatic glucose sensors [11][94][95][96].
The non-enzymatic glucose oxidation catalytic process involves the hemiacetalic hydrogen atom abstraction that occurs in parallel with the adsorption of the organic species [97]. [97] proposed the "incipient hydrous oxide adatom mediator" (IHOAM) model describing the complex electrocatalytic process of glucose. The IHOAM model describes the significance of the “active” hydroxide anions in the domain of the electrode surface produced by the separation of water:(5)H2O→H++OH− to the electro-oxidation of glucose and other organic compounds [92][93]. Moreover, the chemisorption of hydroxide anions to the reductive metal adsorption site (M), results in the production of oxidative adsorbed hydroxide radical (MOHads)
From Equations (5) and (6) it is possible to observe that the MOHadsformation increases by increasing the concentration of OH−. Therefore, non-enzymatic glucose sensing is a pH dependent process, and an highly alkaline environment improves its sensitivity [98].
Several metals, especially noble metals, have been studied as a base material for the electrodes of non-enzymatic glucose biosensors [99][100]. As a result, a deeper understanding of the glucose direct oxidation mechanism was achieved, showing that the mechanism depends directly on the metallic catalyst used in the electrode [75][92][93]. Moreover, advances in material science led to the development of several metal alloys and hybrid materials, allowing for improved properties when compared to noble metals and metal oxides alone [12][101][102][103].
The first peak (potential region 0.15–0.3 V vs. RHE (reversible hydrogen electrode)) corresponds to the hydrogen region and it is characterised by glucose dehydrogenisation leading to glucose adsorption to the electrode surface [97]. The second peak (potential region 0.4–0.8 V vs. RHE) represents the double layer region and it is associated to the water dissociation process (Equation (5)) followed by glucose oxidation that occurs at a lower potential than the required glucose thermodynamic oxidation potential as predicted by the IHOAM model [92][97]. As a result, the glucose oxidation becomes diffusion-controlled, leading to direct bulk glucose oxidation on the oxide layer instead of a surface-bound reaction [92][104].
Noble metals such as Pt and Au, experience a large oxidative current in the double layer region during cathodic scan. Identical anodic currents appear during the cathodic scan for many other organic species, specifically alcohols [105]. Investigations of the produced oxidative currents have demonstrated the current dependence on the glucose concentration [106], pH [107], upper limit potential [98], surface morphology [108], and electrode ion contamination [109].
Strategies to overcome the Pt limitations comprise nanoengineering the Pt surface, fabricating nanocomposite structures, adjusting surface morphology, roughness, and increasing porosity [100][110][111][112][113]. Additionally, the fabrication of nanocomposite Pt-based structures is a widely used approach to improve the catalytic efficiency of noble metals [9][89][98]. This approach reduces production costs and the required amount of Pt and augments the surface catalytic activity by increasing the electrode surface area, evenly dispersing Pt on different substrates such as graphene [114], CNTs [89], and mesoporous carbon [115].
[114] developed a flexible electrochemical glucose sensor using free-standing graphene paper carrying a nanocomposite PtAu alloy and MnO2. The glucose sensor exhibited high sensitivity of 58.54 μA The sensor presented high sensitivity of 30.3 μA [89] developed a non-enzymatic biosensor by electrodepositing Au and Ruthenium (Ru) on the surface of a CNT-based Pt-nanoparticle hybrid composite in a poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) conductive polymer.
[116] demonstrated the potential of using nano porous Pt for non-enzymatic glucose sensing applications and this was followed by several other studies on Pt film electrodes [100][111][112]. [100], developed a prototype of a disposable non-enzymatic blood glucose sensing strip, using nano porous Pt as an electrode material mixed with poly(vinyl acetate) acting as a binding material. The sensor was able to detect glucose in whole human blood with acceptable stability for 30 days and a sensitivity of 0.0054 μAcm−2mgdL−1[100]. [117] selectively dealloyed Si from Pt-Si alloy to create a nano porous Pt electrode with an increase in roughness due to the higher porosity which led to higher glucose sensitivity and lower sensitivity to interfering species such as ascorbic acid.
However, contrary to Pt its glucose oxidation mechanism is still vague, requiring further studies. In this case, the cyclic voltammetry graph only presents two regions, corresponding to the double layer and Au oxide region [118][13][71][107][119]. Moreover, the glucose oxidation is not as dominant in the Au oxide region compared to Pt and mainly occurs in the double layer region where the surface OHadslayers are formed [107]. Results also suggest that high pH levels result in higher faradic current, while at low pH
Different methods such as electrochemical etching and dissolution [101][120][121], electrochemical deposition [122][123][124], and thermal annealing [125] have been used to produce nano porous Au samples aiming to reduce ion contamination and interference with the sensor surface. Verma [101], used Oryza sativa (Asian rice) extract as a reducing agent for the bio-reduction of Au (Au3+) and Ag (Ag+) ions, producing nano precursors leading to the formation of 0D monodispersed tunable nano porous AuNPs. The obtained nano porous AuNPs were then used to modify the surface of a GCE and tested for non-enzymatic glucose sensing using C-V. [120] used magnetron sputtering to fabricate nano porous Au thin films by chemically dealloying the nano porous Au to obtain a 3D bicontinuous ligament nanopore film.
The modified electrode was used to directly detect glucose and was assessed using C-V, showing a linear range from 0.1 to 13 mM, and sensitivity of 0.5 μA mM−1cm−2[124]. [125] developed a new facile, environmentally friendly, cost-effective, and bottom-up approach to obtain a hierarchically porous Au cluster film for direct electrochemical non-enzymatic glucose sensing. The Au-cluster film consisted of a network structure interconnected with Au particles and disordered 3D hierarchical pores. The produced film showed a large surface area, high electrocatalysis, and electroconductivity towards glucose oxidation.
The major advantage of using Au-based electrodes for glucose sensing is the higher current response when compared to Pt-based electrodes, allowing for higher sensitivity and the ability to detect glucose in a neutral pH [107]. However, the main limitation of Au-based electrodes is related to the low glucose oxidation efficiency on the Au electrode surface, especially in the presence of surface OHads[93], which can be reduced by using arrays of nanoelectrodes spaced by non-electroactive materials. Additionally, as these electrodes are better activated in alkaline solutions they cannot be used for in-vivo studies, they suffer from surface contamination from anions such as phosphates and chlorides, and the selectivity is lower than Pt-based electrodes.
Pt and Au are suitable electrode materials for glucose detection but are expensive. Therefore, other non-precious transition metals [126][127] including Nickel (Ni) The redox reaction of transition metals does not follow IHOAM and chemisorption models. The oxidative power of the higher oxide later has enough strength to create surface-bound OHadsradicals, that oxidises organic compounds such as glucose on the electrode surface.
Previous studies highlighted that Cu (II) and Cu (III) couple on the anodic surface of the Cu electrode during glucose electro-oxidation in an alkaline environment [128][129]. Finally, the hydroxyl anions rapidly oxidise the radical intermediate producing gluconolactone [130]. as the CuOOH catalysis requires the presence of hydroxyl anions. Another important disadvantage is related to the competitive ethanol interference which negatively impacts the ability to detect blood glucose level.
[131], developed a portable micro glucose sensor using Cu oxide (CuO) nano-coral arrays (NCA) grown on a nano porous Cu (NPC) electrode. This non-enzymatic sensor showed high catalytic activity of glucose due to the CuO nano-coral arrays and high conductivity due to the NPC. [132] used a wet chemical technique combined with an annealing procedure to produce 3D copper oxide nanowire arrays (CuONWA) on a copper foam (CF) skeleton. The increased sensitivity of this sensor can be attributed to the increase in surface area due to the nanowire arrays as well as the porous copper foam.
In the case of Ni, which exhibits a similar glucose electro-oxidation mechanism to Cu, Ni (II) and Ni (III) couple mediate the glucose redox on the electrode surface. [133] used a femtosecond laser direct writing technique to prepare an Ni foam (NiF). The obtained NiF exhibited a controlled micro and nano superhydrophilicity structure leading to an increased detection area and higher sensitivity (13.822 The nanocomposite was then used to modify the surface of the GCE to detect glucose.
Metal alloys are highly relevant electrode materials due to their high electrocatalysis. The different atoms inside an alloy create a new binding site or reaction pathway that can greatly impact the activation or binding energy of the reagent or intermediate, resulting in a possibly new reaction pathway and reduced overpotential [134]. Novel multi-metallic Pt and Au-based alloys and advances in computational chemistry have allowed development of electrodes with improved catalytic efficiency, stability, and anti-interference [12][102][103].
Several noble metal-based catalysts such as Pt-Ag [113], Pt-Ni [135], Pt-Pb [136], Pt-iridium (Pt-Ir) [137], and Au-Pt [102] were investigated for the development of novel electrodes using electrodeposition or selective dealloying techniques The sensor was used to determine the glucose level in bovine serum albumin samples and showed a linear detection range from 1 to 25 mM and sensitivity of 115.5 The combined use of Pt and Ag resulted in a low overpotential, high sensitivity, and good stability when compared to monometallic-based non-enzymatic glucose sensors. Pt and Ag were also drop casted on the surface of a boron-doped diamond electrode (BDD) resulting in a modified electrode with high stability and selectivity [138].
[139] modified the surface of a Pt electrode using tellurium microtubes through a drop casting method. The sensor exhibited two linear ranges with different sensitivity for each range-first range between 0.1 and 1 mM and sensitivity of 522.61 μA The produced sensor had a linear oxidation current of glucose ranging from 0.1 to 19 mM, sensitivity of 23 μA mM−1cm−2, and the interference from ascorbic acid, uric acid, and fructose was avoided [140].
Metal alloy-based non-enzymatic glucose sensors have great potential in facilitating glucose electro-oxidation with several studies [93] showing its higher sensing performance compared to monometallic-based sensors [141]. The alloy-based sensors typically based on Pt or Au are usually more expensive but present better current response and anti-interference, whilst operating in a neutral pH environment.
This entry is adapted from the peer-reviewed paper 10.3390/s21144672
References
- Ventura, E.E.; Davis, J.N.; Goran, M.I. Sugar content of popular sweetened beverages based on objective laboratory analysis: Focus on fructose content. Obesity 2011, 19, 868–874.
- Conzuelo, F.; Gamella, M.; Campuzano, S.; Ruiz, M.; Reviejo, A.; Pingarron, J. An integrated amperometric biosensor for the determination of lactose in milk and dairy products. J. Agric. Food Chem. 2010, 58, 7141–7148.
- World Health Organization. Leading Causes of Death Worldwide in 2019 (in Millions) 2020, Top 10 Causes of Death. Available online: (accessed on 3 May 2021).
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009.
- Herman, W.H. The global burden of diabetes: An overview. In Diabetes Mellitus in Developing Countries and Underserved Communities; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–5.
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866.
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505.
- Heller, A. Integrated medical feedback systems for drug delivery. AIChE J. 2005, 51, 1054–1066.
- Deshmukh, M.A.; Kang, B.-C.; Ha, T.-J. Non-enzymatic electrochemical glucose sensors based on polyaniline/reduced-graphene-oxide nanocomposites functionalized with silver nanoparticles. J. Mater. Chem. C 2020, 8, 5112–5123.
- Wa, Q.; Xiong, W.; Zhao, R.; He, Z.; Chen, Y.; Wang, X. Nanoscale Ni(OH)x films on carbon cloth prepared by atomic layer deposition and electrochemical activation for glucose sensing. ACS Appl. Nano Mater. 2019, 2, 4427–4434.
- Ma, X.; Tang, K.-l.; Yang, M.; Shi, W.; Zhao, W. Metal–organic framework-derived yolk–shell hollow Ni/ C microspheres for bifunctional non-enzymatic glucose and hydrogen peroxide biosensors. J. Mater. Sci. 2021, 56, 442–456.
- Huo, J.; Lu, L.; Shen, Z.; Gao, H.; Liu, H. Rational design of CoNi alloy and atomic Co/Ni composite as an efficient electrocatalyst. Surf. Innov. 2020, 9, 37–48.
- Gao, X.; Du, X.; Liu, D.; Gao, H.; Wang, P.; Yang, J. Core-shell gold-nickel nanostructures as highly selective and stable nonenzymatic glucose sensor for fermentation process. Sci. Rep. 2020, 10, 1365.
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45.
- Renneberg, R.; Pfeiffer, D.; Lisdat, F.; Wilson, G.; Wollenberger, U.; Ligler, F.; Turner, A.P. Frieder Scheller and the short history of biosensors. In Biosensing for the 21st Century; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–18.
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501.
- Güneş, M.; Karakaya, S.; Dilgin, Y. Development of an interference-minimized amperometric-FIA glucose biosensor at a pyrocatechol violet/glucose dehydrogenase-modified graphite pencil electrode. Chem. Pap. 2020, 74, 1923–1936.
- Cohen, R.; Cohen, Y.; Mukha, D.; Yehezkeli, O. Oxygen insensitive amperometric glucose biosensor based on FAD dependent glucose dehydrogenase co-entrapped with DCPIP or DCNQ in a polydopamine layer. Electrochim. Acta 2021, 367, 137477.
- Iwasa, H.; Hiratsuka, A.; Tanaka, T.; Tsuji, K.; Kishimoto, T.; Watanabe, Y.; Hoshino, Y.; Muguruma, H. Xylose-insensitive direct electron transfer biosensor strip with single-walled carbon nanotubes and novel fungal flavin adenine dinucleotide glucose dehydrogenase. IEEE Sens. J. 2020, 20, 12522–12529.
- Filipiak, M.S.; Vetter, D.; Thodkar, K.; Gutierrez-Sanz, O.; Jönsson-Niedziółka, M.; Tarasov, A. Electron transfer from FAD-dependent glucose dehydrogenase to single-sheet graphene electrodes. Electrochim. Acta 2020, 330, 134998.
- Buford, R.J.; Green, E.C.; McClung, M.J. A microwave frequency sensor for non-invasive blood-glucose measurement. In Proceedings of the 2008 IEEE Sensors Applications Symposium, Atlanta, GA, USA, 12–14 February 2008; pp. 4–7.
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R.M. Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: Novel design utilizing a four-cell CSRR hexagonal configuration. Sci. Rep. 2020, 10, 15200.
- Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Wearable non-invasive monitors of diabetes and hypoxia through continuous analysis of sweat. Talanta 2020, 215, 120922.
- Alam, M.M.; Saha, S.; Saha, P.; Nur, F.N.; Moon, N.N.; Karim, A.; Azam, S. D-care: A non-invasive glucose measuring technique for monitoring diabetes patients. In Proceedings of the International Joint Conference on Computational Intelligence, Dhaka, India, 25–26 October 2020; pp. 443–453.
- Updike, S.; Hicks, G. The enzyme electrode. Nature 1967, 214, 986–988.
- Guilbault, G.; Lubrano, G. An enzyme electrode for the amperometric determination of glucose. Anal. Chim. Acta 1973, 64, 439–455.
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491.
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. Prussian blue-based first-generation biosensor. A sensitive amperometric electrode for glucose. Anal. Chem. 1995, 67, 2419–2423.
- Kaçar, C.; Dalkiran, B.; Erden, P.E.; Kiliç, E. An amperometric hydrogen peroxide biosensor based on Co3O4 nanoparticles and multiwalled carbon nanotube modified glassy carbon electrode. Appl. Surf. Sci. 2014, 311, 139–146.
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825.
- Poulos, N.G.; Hall, J.R.; Leopold, M.C. Functional layer-by-layer design of xerogel-based first-generation amperometric glucose biosensors. Langmuir 2015, 31, 1547–1555.
- Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis 2001, 13, 983–988.
- Mǎdǎraş, M.B.; Buck, R.P. Miniaturized biosensors employing electropolymerized permselective films and their use for creatinine assays in human serum. Anal. Chem. 1996, 68, 3832–3839.
- Palmisano, F.; Malitesta, C.; Centonze, D.; Zambonin, P. Correlation between permselectivity and chemical structure of overoxidized polypyrrole membranes used in electroproduced enzyme biosensors. Anal. Chem. 1995, 67, 2207–2211.
- Jeerapan, I.; Sempionatto, J.R.; You, J.-M.; Wang, J. Enzymatic glucose/oxygen biofuel cells: Use of oxygen-rich cathodes for operation under severe oxygen-deficit conditions. Biosens. Bioelectron. 2018, 122, 284–289.
- Sasso, S.V.; Pierce, R.J.; Walla, R.; Yacynych, A.M. Electropolymerized 1, 2-diaminobenzene as a means to prevent interferences and fouling and to stabilize immobilized enzyme in electrochemical biosensors. Anal. Chem. 1990, 62, 1111–1117.
- Malitesta, C.; Palmisano, F.; Torsi, L.; Zambonin, P.G. Glucose fast-response amperometric sensor based on glucose oxidase immobilized in an electropolymerized poly (o-phenylenediamine) film. Anal. Chem. 1990, 62, 2735–2740.
- Zhang, Y.; Hu, Y.; Wilson, G.S.; Moatti-Sirat, D.; Poitout, V.; Reach, G. Elimination of the acetaminophen interference in an implantable glucose sensor. Anal. Chem. 1994, 66, 1183–1188.
- Emr, S.A.; Yacynych, A.M. Use of polymer films in amperometric biosensors. Electroanalysis 1995, 7, 913–923.
- Wang, J.; Wu, H. Highly selective biosensing of glucose utilizing a glucose oxidase+ rhodium+ Nafion® biocatalytic-electrocatalytic-permselective surface microstructure. J. Electroanal. Chem. 1995, 395, 287–291.
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104.
- Tang, H.; Yan, F.; Lin, P.; Xu, J.; Chan, H.L.W. Highly sensitive glucose biosensors based on organic electrochemical transistors using platinum gate electrodes modified with enzyme and nanomaterials. Adv. Funct. Mater. 2011, 21, 2264–2272.
- Baek, S.H.; Roh, J.; Park, C.Y.; Kim, M.W.; Shi, R.; Kailasa, S.K.; Park, T.J. Cu-nanoflower decorated gold nanoparticles-graphene oxide nanofiber as electrochemical biosensor for glucose detection. Mater. Sci. Eng. C 2020, 107, 110273.
- Mei, Q.; Fu, R.; Ding, Y.; Wang, A.; Duan, D.; Ye, D. Electrospinning of highly dispersed Ni/CoO carbon nanofiber and its application in glucose electrochemical sensor. J. Electroanal. Chem. 2019, 847, 113075.
- Reach, G.; Wilson, G.S. Can continuous glucose monitoring be used for the treatment of diabetes. Anal. Chem. 1992, 64, 381A–386A.
- Sekretaryova, A.N.; Vokhmyanina, D.V.; Chulanova, T.O.; Karyakina, E.E.; Karyakin, A.A. Reagentless biosensor based on glucose oxidase wired by the mediator freely diffusing in enzyme containing membrane. Anal. Chem. 2012, 84, 1220–1223.
- Wang, J.; Lu, F. Oxygen-rich oxidase enzyme electrodes for operation in oxygen-free solutions. J. Am. Chem. Soc. 1998, 120, 1048–1050.
- Wang, J.; Mo, J.-W.; Li, S.; Porter, J. Comparison of oxygen-rich and mediator-based glucose-oxidase carbon-paste electrodes. Anal. Chim. Acta 2001, 441, 183–189.
- Kontani, R.; Tsujimura, S.; Kano, K. Air diffusion biocathode with CueO as electrocatalyst adsorbed on carbon particle-modified electrodes. Bioelectrochemistry 2009, 76, 10–13.
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-based detection of ketone bodies along with glucose and lactate: Toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal. Chem. 2019, 92, 2291–2300.
- Schuhmann, W.; Ohara, T.J.; Schmidt, H.L.; Heller, A. Electron transfer between glucose oxidase and electrodes via redox mediators bound with flexible chains to the enzyme surface. J. Am. Chem. Soc. 1991, 113, 1394–1397.
- Ozoemena, K.I.; Nyokong, T. Novel amperometric glucose biosensor based on an ether-linked cobalt (II) phthalocyanine–cobalt (II) tetraphenylporphyrin pentamer as a redox mediator. Electrochim. Acta 2006, 51, 5131–5136.
- Dervisevic, M.; Cevik, E.; Şenel, M. Development of glucose biosensor based on reconstitution of glucose oxidase onto polymeric redox mediator coated pencil graphite electrodes. Enzym. Microb. Technol. 2015, 68, 69–76.
- Al-Sagur, H.; Komathi, S.; Khan, M.A.; Gurek, A.G.; Hassan, A. A novel glucose sensor using lutetium phthalocyanine as redox mediator in reduced graphene oxide conducting polymer multifunctional hydrogel. Biosens. Bioelectron. 2017, 92, 638–645.
- Ahmed, M.U.; Hossain, M.M.; Tamiya, E. Electrochemical biosensors for medical and food applications. Electroanalysis 2008, 20, 616–626.
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709.
- Suzuki, N.; Lee, J.; Loew, N.; Takahashi-Inose, Y.; Okuda-Shimazaki, J.; Kojima, K.; Mori, K.; Tsugawa, W.; Sode, K. Engineered glucose oxidase capable of quasi-direct electron transfer after a quick-and-easy modification with a mediator. Int. J. Mol. Sci. 2020, 21, 1137.
- Lee, J.; Hyun, K.; Kwon, Y. A study on the stability and sensitivity of mediator-based enzymatic glucose sensor measured by catalyst consisting of multilayer stacked via layer-by-layer. J. Ind. Eng. Chem. 2021, 93, 383–387.
- Boussema, F.; Gross, A.J.; Hmida, F.; Ayed, B.; Majdoub, H.; Cosnier, S.; Maaref, A.; Holzinger, M. Dawson-type polyoxometalate nanoclusters confined in a carbon nanotube matrix as efficient redox mediators for enzymatic glucose biofuel cell anodes and glucose biosensors. Biosens. Bioelectron. 2018, 109, 20–26.
- Marquitan, M.; Bobrowski, T.; Ernst, A.; Wilde, P.; Clausmeyer, J.; Ruff, A.; Schuhmann, W. Miniaturized amperometric glucose sensors based on polymer/enzyme modified carbon electrodes in the sub-micrometer scale. J. Electrochem. Soc. 2018, 165, G3008.
- Bobrowski, T.; Schuhmann, W. Long-term implantable glucose biosensors. Curr. Opin. Electrochem. 2018, 10, 112–119.
- Heller, A. Electrical connection of enzyme redox centers to electrodes. J. Phys. Chem. 1992, 96, 3579–3587.
- Gallay, P.A.; Rubianes, M.D.; Gutierrez, F.A.; Rivas, G.A. Avidin and Glucose Oxidase-non-covalently Functionalized Multi-walled Carbon Nanotubes: A New Analytical Tool for Building a Bienzymatic Glucose Biosensor. Electroanalysis 2019, 31, 1888–1894.
- Rafighi, P.; Tavahodi, M.; Haghighi, B. Fabrication of a third-generation glucose biosensor using graphene-polyethyleneimine-gold nanoparticles hybrid. Sens. Actuators B Chem. 2016, 232, 454–461.
- Mehmeti, E.; Stanković, D.M.; Chaiyo, S.; Zavasnik, J.; Žagar, K.; Kalcher, K. Wiring of glucose oxidase with graphene nanoribbons: An electrochemical third generation glucose biosensor. Microchim. Acta 2017, 184, 1127–1134.
- Willner, I.; Heleg-Shabtai, V.; Blonder, R.; Katz, E.; Tao, G.; Bückmann, A.F.; Heller, A. Electrical wiring of glucose oxidase by reconstitution of FAD-modified monolayers assembled onto Au-electrodes. J. Am. Chem. Soc. 1996, 118, 10321–10322.
- Fruk, L.; Kuo, C.H.; Torres, E.; Niemeyer, C.M. Apoenzyme reconstitution as a chemical tool for structural enzymology and biotechnology. Angew. Chem. Int. Ed. 2009, 48, 1550–1574.
- Zayats, M.; Katz, E.; Baron, R.; Willner, I. Reconstitution of apo-glucose dehydrogenase on pyrroloquinoline quinone-functionalized Au nanoparticles yields an electrically contacted biocatalyst. J. Am. Chem. Soc. 2005, 127, 12400–12406.
- Şenel, M.; Nergiz, C.; Dervisevic, M.; Çevik, E. Development of amperometric glucose biosensor based on reconstitution of glucose oxidase on polymeric 3-aminophenyl boronic acid monolayer. Electroanalysis 2013, 25, 1194–1200.
- Degani, Y.; Heller, A. Electrical communication between redox centers of glucose oxidase and electrodes via electrostatically and covalently bound redox polymers. J. Am. Chem. Soc. 1989, 111, 2357–2358.
- Holland, J.T.; Lau, C.; Brozik, S.; Atanassov, P.; Banta, S. Engineering of glucose oxidase for direct electron transfer via site-specific gold nanoparticle conjugation. J. Am. Chem. Soc. 2011, 133, 19262–19265.
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-based biosensors: Recent trends, challenges and future perspectives. Biosens. Bioelectron. 2019, 141, 111435.
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173.
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.-H.; Ahn, S.J.; Lee, J.-J. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 2010, 10, 4855–4886.
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022.
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: Recent progress, applications, and future perspective. Chem. Rev. 2018, 119, 120–194.
- Buk, V.; Pemble, M.E. A highly sensitive glucose biosensor based on a micro disk array electrode design modified with carbon quantum dots and gold nanoparticles. Electrochim. Acta 2019, 298, 97–105.
- Shrestha, B.K.; Ahmad, R.; Shrestha, S.; Park, C.H.; Kim, C.S. Globular shaped polypyrrole doped well-dispersed functionalized multiwall carbon nanotubes/nafion composite for enzymatic glucose biosensor application. Sci. Rep. 2017, 7, 16191.
- Li, J.; Liu, Y.; Tang, X.; Xu, L.; Min, L.; Xue, Y.; Hu, X.; Yang, Z. Multiwalled carbon nanotubes coated with cobalt (II) sulfide nanoparticles for electrochemical sensing of glucose via direct electron transfer to glucose oxidase. Microchim. Acta 2020, 187, 80.
- Hao, L.; Li, S.-S.; Wang, J.; Tan, Y.; Bai, L.; Liu, A. MnO2/multi-walled carbon nanotubes based nanocomposite with enhanced electrocatalytic activity for sensitive amperometric glucose biosensing. J. Electroanal. Chem. 2020, 878, 114602.
- Vukojević, V.; Djurdjić, S.; Ognjanović, M.; Fabian, M.; Samphao, A.; Kalcher, K.; Stanković, D.M. Enzymatic glucose biosensor based on manganese dioxide nanoparticles decorated on graphene nanoribbons. J. Electroanal. Chem. 2018, 823, 610–616.
- Mao, Q.; Jing, W.; Zhou, F.; Liu, S.; Gao, W.; Wei, Z.; Jiang, Z. Depositing reduced graphene oxide on ZnO nanorods to improve the performance of enzymatic glucose sensors. Mater. Sci. Semicond. Process. 2021, 121, 105391.
- Hossain, M.F.; Slaughter, G. PtNPs decorated chemically derived graphene and carbon nanotubes for sensitive and selective glucose biosensing. J. Electroanal. Chem. 2020, 861, 113990.
- Kim, I.; Kwon, D.; Lee, D.; Lee, T.H.; Lee, J.H.; Lee, G.; Yoon, D.S. A highly permselective electrochemical glucose sensor using red blood cell membrane. Biosens. Bioelectron. 2018, 102, 617–623.
- Aydemir, N.; Malmström, J.; Travas-Sejdic, J. Conducting polymer based electrochemical biosensors. Phys. Chem. Chem. Phys. 2016, 18, 8264–8277.
- Xu, M.; Song, Y.; Ye, Y.; Gong, C.; Shen, Y.; Wang, L.; Wang, L. A novel flexible electrochemical glucose sensor based on gold nanoparticles/polyaniline arrays/carbon cloth electrode. Sens. Actuators B Chem. 2017, 252, 1187–1193.
- Soylemez, S.; Kaya, H.Z.; Udum, Y.A.; Toppare, L. A multipurpose conjugated polymer: Electrochromic device and biosensor construction for glucose detection. Org. Electron. 2019, 65, 327–333.
- Wang, L.; Xu, M.; Xie, Y.; Qian, C.; Ma, W.; Wang, L.; Song, Y. Ratiometric electrochemical glucose sensor based on electroactive Schiff base polymers. Sens. Actuators B Chem. 2019, 285, 264–270.
- Nguyen, T.N.H.; Jin, X.; Nolan, J.K.; Xu, J.; Le, K.V.H.; Lam, S.; Wang, Y.; Alam, M.A.; Lee, H. Printable Nonenzymatic Glucose Biosensors Using Carbon Nanotube-PtNP Nanocomposites Modified with AuRu for Improved Selectivity. ACS Biomater. Sci. Eng. 2020, 6, 5315–5325.
- Vassilyev, Y.B.; Khazova, O.A.; Nikolaeva, N.N. Kinetics and mechanism of glucose electrooxidation on different electrode-catalysts: Part I. Adsorption and oxidation on platinum. J. Electroanal. Chem. Interfacial Electrochem. 1985, 196, 105–125.
- Vassilyev, Y.B.; Khazova, O.A.; Nikolaeva, N.N. Kinetics and mechanism of glucose electrooxidation on different electrode-catalysts: Part II. Effect of the nature of the electrode and the electrooxidation mechanism. J. Electroanal. Chem. Interfacial Electrochem. 1985, 196, 127–144.
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301.
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34.
- Ci, S.; Huang, T.; Wen, Z.; Cui, S.; Mao, S.; Steeber, D.A.; Chen, J. Nickel oxide hollow microsphere for non-enzyme glucose detection. Biosens. Bioelectron. 2014, 54, 251–257.
- Fu, S.; Fan, G.; Yang, L.; Li, F. Non-enzymatic glucose sensor based on Au nanoparticles decorated ternary Ni-Al layered double hydroxide/single-walled carbon nanotubes/graphene nanocomposite. Electrochim. Acta 2015, 152, 146–154.
- Choi, T.; Kim, S.H.; Lee, C.W.; Kim, H.; Choi, S.-K.; Kim, S.-H.; Kim, E.; Park, J.; Kim, H. Synthesis of carbon nanotube–nickel nanocomposites using atomic layer deposition for high-performance non-enzymatic glucose sensing. Biosens. Bioelectron. 2015, 63, 325–330.
- Burke, L.D. Premonolayer oxidation and its role in electrocatalysis. Electrochim. Acta 1994, 39, 1841–1848.
- Şavk, A.; Aydın, H.; Cellat, K.; Şen, F. A novel high performance non-enzymatic electrochemical glucose biosensor based on activated carbon-supported Pt-Ni nanocomposite. J. Mol. Liq. 2020, 300, 112355.
- Eryiğit, M.; Çepni, E.; Urhan, B.K.; Doğan, H.Ö.; Özer, T.Ö. Nonenzymatic glucose sensor based on poly (3, 4-ethylene dioxythiophene)/electroreduced graphene oxide modified gold electrode. Synth. Met. 2020, 268, 116488.
- Lee, S.; Lee, J.; Park, S.; Boo, H.; Kim, H.C.; Chung, T.D. Disposable non-enzymatic blood glucose sensing strip based on nanoporous platinum particles. Appl. Mater. Today 2018, 10, 24–29.
- Verma, N. A green synthetic approach for size tunable nanoporous gold nanoparticles and its glucose sensing application. Appl. Surf. Sci. 2018, 462, 753–759.
- Wang, J.; Zhao, D.; Xu, C. Nonenzymatic electrochemical sensor for glucose based on nanoporous platinum-gold alloy. J. Nanosci. Nanotechnol. 2016, 16, 7145–7150.
- Jafarian, M.; Forouzandeh, F.; Danaee, I.; Gobal, F.; Mahjani, M. Electrocatalytic oxidation of glucose on Ni and NiCu alloy modified glassy carbon electrode. J. Solid State Electrochem. 2009, 13, 1171–1179.
- Skou, E. The electrochemical oxidation of glucose on platinum—I. The oxidation in 1 M H2SO4. Electrochim. Acta 1977, 22, 313–318.
- Zhao, Y.; Li, X.; Schechter, J.M.; Yang, Y. Revisiting the oxidation peak in the cathodic scan of the cyclic voltammogram of alcohol oxidation on noble metal electrodes. RSC Adv. 2016, 6, 5384–5390.
- Xu, Q.; Yin, L.; Hou, C.; Liu, X.; Hu, X. Facile fabrication of nanoporous platinum by alloying–dealloying process and its application in glucose sensing. Sens. Actuators B Chem. 2012, 173, 716–723.
- Pasta, M.; La Mantia, F.; Cui, Y. Mechanism of glucose electrochemical oxidation on gold surface. Electrochim. Acta 2010, 55, 5561–5568.
- Hu, Y.; He, F.; Ben, A.; Chen, C. Synthesis of hollow Pt–Ni–graphene nanostructures for nonenzymatic glucose detection. J. Electroanal. Chem. 2014, 726, 55–61.
- Mohammed, F.A.; Khalaf, M.M.; Mohamed, I.M.; Saleh, M.; Abd El-Lateef, H.M. Synthesis of mesoporous nickel ferrite nanoparticles by use of citrate framework methodology and application for electrooxidation of glucose in alkaline media. Microchem. J. 2020, 153, 104507.
- Unmüssig, T.; Weltin, A.; Urban, S.; Daubinger, P.; Urban, G.A.; Kieninger, J. Non-enzymatic glucose sensing based on hierarchical platinum micro-/nanostructures. J. Electroanal. Chem. 2018, 816, 215–222.
- Weremfo, A.; Fong, S.T.C.; Khan, A.; Hibbert, D.B.; Zhao, C. Electrochemically roughened nanoporous platinum electrodes for non-enzymatic glucose sensors. Electrochim. Acta 2017, 231, 20–26.
- Hu, Y.; Niu, X.; Zhao, H.; Tang, J.; Lan, M. Enzyme-free amperometric detection of glucose on platinum-replaced porous copper frameworks. Electrochim. Acta 2015, 165, 383–389.
- Lin, K.-C.; Yang, C.-Y.; Chen, S.-M. Fabrication of a nonenzymatic glucose sensor based on multi-walled carbon nanotubes decorated with platinum and silver hybrid composite. Int. J. Electrochem. Sci 2015, 10, 3726–3737.
- Xiao, F.; Li, Y.; Gao, H.; Ge, S.; Duan, H. Growth of coral-like PtAu–MnO2 binary nanocomposites on free-standing graphene paper for flexible nonenzymatic glucose sensors. Biosens. Bioelectron. 2013, 41, 417–423.
- Zhang, C.; Zhang, R.; Gao, X.; Cheng, C.; Hou, L.; Li, X.; Chen, W. Small naked Pt nanoparticles confined in mesoporous shell of hollow carbon spheres for high-performance nonenzymatic sensing of H2O2 and glucose. ACS Omega 2018, 3, 96–105.
- Park, S.; Chung, T.D.; Kim, H.C. Nonenzymatic glucose detection using mesoporous platinum. Anal. Chem. 2003, 75, 3046–3049.
- Kim, S.H.; Choi, J.B.; Nguyen, Q.N.; Lee, J.M.; Park, S.; Chung, T.D.; Byun, J.Y. Nanoporous platinum thin films synthesized by electrochemical dealloying for nonenzymatic glucose detection. Phys. Chem. Chem. Phys. 2013, 15, 5782–5787.
- Casella, I.G.; Destradis, A.; Desimoni, E. Colloidal gold supported onto glassy carbon substrates as an amperometric sensor for carbohydrates in flow injection and liquid chromatography. Analyst 1996, 121, 249–254.
- Burke, L.; Ryan, T. The role of incipient hydrous oxides in the oxidation of glucose and some of its derivatives in aqueous media. Electrochim. Acta 1992, 37, 1363–1370.
- Xue, Y.; Wang, S.; Shi, P.; Huang, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Denis, P.; Fecht, H.-J. Nanoporous gold chemically de-alloyed from Au-based amorphous thin film for electrochemical nonenzymatic H2O2 sensing. Chem. Phys. Lett. 2019, 723, 22–27.
- Chen, A.; Wang, J.; Wang, Y.; Jia, Y.; Gu, J.; Xie, X.; Pan, D. Effects of pore size and residual Ag on electrocatalytic properties of nanoporous gold films prepared by pulse electrochemical dealloying. Electrochim. Acta 2015, 153, 552–558.
- Bai, Y.; Yang, W.; Sun, Y.; Sun, C. Enzyme-free glucose sensor based on a three-dimensional gold film electrode. Sens. Actuators B Chem. 2008, 134, 471–476.
- Cherevko, S.; Chung, C.-H. Gold nanowire array electrode for non-enzymatic voltammetric and amperometric glucose detection. Sens. Actuators B Chem. 2009, 142, 216–223.
- Sanzó, G.; Taurino, I.; Antiochia, R.; Gorton, L.; Favero, G.; Mazzei, F.; De Micheli, G.; Carrara, S. Bubble electrodeposition of gold porous nanocorals for the enzymatic and non-enzymatic detection of glucose. Bioelectrochemistry 2016, 112, 125–131.
- Han, L.; Zhang, S.; Han, L.; Yang, D.-P.; Hou, C.; Liu, A. Porous gold cluster film prepared from BSA microspheres for electrochemical nonenzymatic glucose sensor. Electrochim. Acta 2014, 138, 109–114.
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, Y. Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: Opportunities and challenges. RSC Adv. 2016, 6, 84893–84905.
- Tee, S.Y.; Teng, C.P.; Ye, E. Metal nanostructures for non-enzymatic glucose sensing. Mater. Sci. Eng. C 2017, 70, 1018–1030.
- Niu, X.; Li, Y.; Tang, J.; Hu, Y.; Zhao, H.; Lan, M. Electrochemical sensing interfaces with tunable porosity for nonenzymatic glucose detection: A Cu foam case. Biosens. Bioelectron. 2014, 51, 22–28.
- Riman, D.; Spyrou, K.; Karantzalis, A.E.; Hrbac, J.; Prodromidis, M.I. Glucose sensing on graphite screen-printed electrode modified by sparking of copper nickel alloys. Talanta 2017, 165, 466–473.
- Chen, X.; Tian, X.; Zhao, L.; Huang, Z.; Oyama, M. Nonenzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with graphene nanosheets and Pt-Pd bimetallic nanocubes. Microchim. Acta 2014, 181, 783–789.
- Chen, H.; Fan, G.; Zhao, J.; Qiu, M.; Sun, P.; Fu, Y.; Han, D.; Cui, G. A portable micro glucose sensor based on copper-based nanocomposite structure. New J. Chem. 2019, 43, 7806–7813.
- Liu, X.; Yang, W.; Chen, L.; Jia, J. Three-dimensional copper foam supported CuO nanowire arrays: An efficient non-enzymatic glucose sensor. Electrochim. Acta 2017, 235, 519–526.
- Gao, X.; Feng, W.; Zhu, Z.; Wu, Z.; Li, S.; Kan, S.; Qiu, X.; Guo, A.; Chen, W.; Yin, K. Rapid Fabrication of Superhydrophilic Micro/Nanostructured Nickel Foam Toward High-Performance Glucose Sensor. Adv. Mater. Interfaces 2021, 8, 2002133.
- Aoun, S.B.; Dursun, Z.; Koga, T.; Bang, G.S.; Sotomura, T.; Taniguchi, I. Effect of metal ad-layers on Au (1 1 1) electrodes on electrocatalytic oxidation of glucose in an alkaline solution. J. Electroanal. Chem. 2004, 567, 175–183.
- Zhao, Y.; Fan, L.; Hong, B.; Ren, J.; Zhang, M.; Que, Q.; Ji, J. Nonenzymatic detection of glucose using three-dimensional PtNi nanoclusters electrodeposited on the multiwalled carbon nanotubes. Sens. Actuators B Chem. 2016, 231, 800–810.
- Xiao, F.; Zhao, F.; Mei, D.; Mo, Z.; Zeng, B. Nonenzymatic glucose sensor based on ultrasonic-electrodeposition of bimetallic PtM (M = Ru, Pd and Au) nanoparticles on carbon nanotubes–ionic liquid composite film. Biosens. Bioelectron. 2009, 24, 3481–3486.
- Li, J.; Koinkar, P.; Fuchiwaki, Y.; Yasuzawa, M. A fine pointed glucose oxidase immobilized electrode for low-invasive amperometric glucose monitoring. Biosens. Bioelectron. 2016, 86, 90–94.
- Nantaphol, S.; Watanabe, T.; Nomura, N.; Siangproh, W.; Chailapakul, O.; Einaga, Y. Bimetallic Pt–Au nanocatalysts electrochemically deposited on boron-doped diamond electrodes for nonenzymatic glucose detection. Biosens. Bioelectron. 2017, 98, 76–82.
- Guascito, M.R.; Chirizzi, D.; Malitesta, C.; Siciliano, M.; Siciliano, T.; Tepore, A. Amperometric non-enzymatic bimetallic glucose sensor based on platinum tellurium microtubes modified electrode. Electrochem. Commun. 2012, 22, 45–48.
- Guo, T.; Zhang, Y.; Ouyang, Y.; Yu, G.; Liao, Y.; Zhang, Z. Facile Synthesis of Bimetallic PtxCu1-x Nanostrands and Their Application in Non-Enzymatic Glucose Sensor. Int. J. Electrochem. Sci. 2016, 11, 6477–6490.
- Pandey, P.C.; Panday, D.; Pandey, G. 3-Aminopropyltrimethoxysilane and organic electron donors mediated synthesis of functional amphiphilic gold nanoparticles and their bioanalytical applications. RSC Adv. 2014, 4, 60563–60572.
