Infertility (defined as the inability to conceive within a year of unprotected coitus) is a global health and social issue affecting close to 15% of couples. In half of the couples seeking medical treatment for infertility, male factor infertility is identified together with abnormal semen characteristics. The focus of current clinical practice is laid on the presence of sufficient sperm count in the ejaculated specimen with adequate motility and morphology of spermatozoa capable of giving fertilization a chance (conventional semen analysis).
- male infertility
- DNA breaks
- genome instability
1. Overview
Genome instability may play a role in severe cases of male infertility, with disrupted spermatogenesis being just one manifestation of decreased general health and increased morbidity. Here, we review the data on the association of male infertility with genetic, epigenetic, and environmental alterations, the causes and consequences, and the methods for assessment of genome instability. Male infertility research has provided evidence that spermatogenic defects are often not limited to testicular dysfunction. An increased incidence of urogenital disorders and several types of cancer, as well as overall reduced health (manifested by decreased life expectancy and increased morbidity) have been reported in infertile men. The pathophysiological link between decreased life expectancy and male infertility supports the notion of male infertility being a systemic rather than an isolated condition. It is driven by the accumulation of DNA strand breaks and premature cellular senescence. We have presented extensive data supporting the notion that genome instability can lead to severe male infertility termed “idiopathic oligo-astheno-teratozoospermia.” We have detailed that genome instability in men with oligo-astheno-teratozoospermia (OAT) might depend on several genetic and epigenetic factors such as chromosomal heterogeneity, aneuploidy, micronucleation, dynamic mutations, RT, PIWI/piRNA regulatory pathway, pathogenic allelic variants in repair system genes, DNA methylation, environmental aspects, and lifestyle factors.
2. Infertility
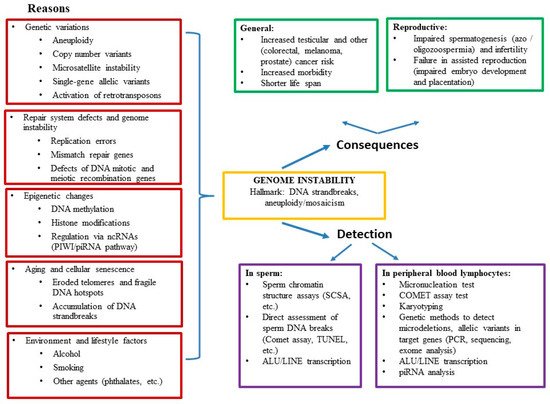
3. Conclusions
In summary, there are extensive data supporting the notion that genome instability may lead to severe male infertility (OAT), whereas genetic tests recommended for routine clinical investigation (such as testing for karyotype, Y chromosome AZF region microdeletions) provide a diagnosis in only 15–25% of cases. We have detailed that genome instability in men with OAT depends on several other genetic and epigenetic factors such as chromosomal heterogeneity, aneuploidy, micronucleation, dynamic mutations, RT, PIWI/piRNA regulatory pathway, pathogenic allelic variants in repair system genes, DNA methylation, and environmental aspects (e.g., smoking, alcohol).
This entry is adapted from the peer-reviewed paper 10.3390/life11070628
References
- Nieschlag, E. Scope and Goals of Andrology. In Andrology: Male Reproductive Health and Dysfunction; Nieschlag, E., Behre, H.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–8.
- Punab, M.; Poolamets, O.; Paju, P.; Vihljajev, V.; Pomm, K.; Ladva, R.; Korrovits, P.; Laan, M. Causes of male infertility: A 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum. Reprod. 2016, 32, 18–31.
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613.
- Sengupta, P.; Borges, E.; Dutta, S.; Krajewska-Kułak, E. Decline in sperm count in European men during the past 50 years. Hum. Exp. Toxicol. 2017, 37, 247–255.
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm Morphology, Motility, and Concentration in Fertile and Infertile Men. New Engl. J. Med. 2001, 345, 1388–1393.
- Cao, X.W.; Lin, K.; Li, C.Y.; Yuan, C.W. A review of WHO Laboratory Manual for the Examination and Processing of Human Semen (5th edition)]. Zhonghua Nan Ke Xue 2011, 17, 1059–1063.
- van den Hoven, L.; Hendriks, J.C.; Verbeet, J.G.; Westphal, J.R.; Wetzels, A.M. Status of sperm morphology assessment: An evaluation of methodology and clinical value. Fertil. Steril. 2015, 103, 53–58.
- O’Neill, C.L.; Parrella, A.; Keating, D.; Cheung, S.; Rosenwaks, Z.; Palermo, G.D. A treatment algorithm for couples with unexplained infertility based on sperm chromatin assessment. J. Assist. Reprod. Genet. 2018, 35, 1911–1917.
- Evenson, D.P.; Jost, L.K.; Marshall, D.; Zinaman, M.J.; Clegg, E.; Purvis, K.; De Angelis, P.; Claussen, O.P. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum. Reprod. 1999, 14, 1039–1049.
- Bungum, M.; Humaidan, P.; Axmon, A.; Spano, M.; Bungum, L.; Erenpreiss, J.; Giwercman, A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum. Reprod. 2007, 22, 174–179.
- Erenpreiss, J.; Je, E.; Giwercman, A.; Tsarev, I.; Erenpreisa, J.; Spano, M. Toluidine blue cytometry test for sperm DNA conformation: Comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum. Reprod. 2004, 19, 2277–2282.
- Tsarev, I.; Bungum, M.; Giwercman, A.; Erenpreisa, J.; Ebessen, T.; Ernst, E.; Erenpreiss, J. Evaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessment. Hum. Reprod. 2009, 24, 1569–1574.
- Kim, H.-S.; Kang, M.J.; Kim, S.A.; Oh, S.K.; Kim, H.; Ku, S.-Y.; Kim, S.H.; Moon, S.Y.; Choi, Y.M. The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clin. Exp. Reprod. Med. 2013, 40, 23–28.
- Erenpreiss, J.; Bars, J.; Lipatnikova, V.; Erenpreisa, J.; Zalkalns, J. Comparative study of cytochemical tests for sperm chromatin integrity. J. Androl. 2001, 22, 45–53.
- Erenpreiss, J.; Spano, M.; Bungum, M.; Giwercman, A. Sperm chromatin structure and male fertility: Biological and clinical aspects. Asian J. Androl. 2006, 8, 11–29.
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 30, 120–127.
- Pourmasumi, S.; Khoradmehr, A.; Rahiminia, T.; Sabeti, P.; Talebi, A.R.; Ghasemzadeh, J. Evaluation of Sperm Chromatin Integrity Using Aniline Blue and Toluidine Blue Staining in Infertile and Normozoospermic Men. J. Reprod. Infertil. 2019, 20, 95–101.
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881.
- Collins, J.A.; Barnhart, K.T.; Schlegel, P.N. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil. Steril. 2008, 89, 823–831.
- Lewis, S.E.; Aitken, R.J.; Conner, S.J.; De Iuliis, G.; Evenson, D.P.; Henkel, R.; Giwercman, A.; Gharagozloo, P. The impact of sperm DNA damage in assisted conception and beyond: Recent advances in diagnosis and treatment. Reprod. Biomed. Online 2013, 27, 325–337.
- Aguilera, A.; Gómez-González, B. Genome instability: A mechanistic view of its causes and consequences. Nat. Rev. Genet. 2008, 9, 204–217.
- Vijg, J.; Montagna, C. Genome instability and aging: Cause or effect? Transl. Med. Aging 2017, 1, 5–11.
- Iourov, I.Y.; Vorsanova, S.G.; Yurov, Y.B.; Kutsev, S.I. Ontogenetic and Pathogenetic Views on Somatic Chromosomal Mosaicism. Genes 2019, 10, 379.
- Jensen, T.K.; Jacobsen, R.; Christensen, K.; Nielsen, N.C.; Bostofte, E. Good Semen Quality and Life Expectancy: A Cohort Study of 43,277 Men. Am. J. Epidemiol. 2009, 170, 559–565.
- Salonia, A.; Matloob, R.; Gallina, A.; Abdollah, F.; Saccà, A.; Briganti, A.; Suardi, N.; Colombo, R.; Rocchini, L.; Guazzoni, G.F.; et al. Are Infertile Men Less Healthy than Fertile Men? Results of a Prospective Case-Control Survey. Eur. Urol. 2009, 56, 1025–1032.
- Aston, K.I.; Carrell, D.T. Emerging evidence for the role of genomic instability in male factor infertility. Syst. Biol. Reprod. Med. 2011, 58, 71–80.
- Cheung, S.; Parrella, A.; Rosenwaks, Z.; Palermo, G.D. Genetic and epigenetic profiling of the infertile male. PLoS ONE 2019, 14, e0214275.
