Archaea, and thereby, Sulfolobus spp. exhibit a unique lipid composition of ether lipids, which are altered in regard to the ratio of diether to tetraether lipids, number of cyclopentane rings and type of head groups, as a coping mechanism against environmental changes. Sulfolobales mainly consist of C40-40 tetraether lipids (caldarchaeol) and partly of C20-20 diether lipids (archaeol). A variant of caldarchaeol called glycerol dialkylnonitol tetraether (GDNT) has only been found in Sulfolobus and other members of the Creanarchaeota phylum so far. Altering the numbers of incorporated cyclopentane rings or the the diether to tetraether ratio results in more tightly packed membranes or vice versa.
- Sulfolobus
- archaea
- membrane
- tetraether
- diether
- lipids
1. The Cell Membrane and Lipids of Sulfolobus spp.
The thermoacidophilic genus Sulfolobus belongs to the phylum Crenarchaeota and is a promising player for biotechnology [1], since it harbors a couple of valuable products, such as extremozymes [2], trehalose [3], archaeocins [4] and lipids for producing archaeosomes [5]. Genetic tools for this genus have rapidly advanced in recent years [6], generating new possibilities in basic science and for biotechnological applications alike. The cultivation conditions are preferably at around 80 °C and pH 3 [7]. Sulfolobus species are able to grow aerobically and can be readily cultivated on a laboratory scale. These organisms became a model organism for Crenarchaeota and for adaption processes to extreme environments [8][9][10][11][12][13]. Sulfolobus species were found in solfataric fields all over the world [14].
A major drawback of cultivating this organism was the lack of a defined cultivation medium. Until 2019, the “Brock Medium” served as the prevalent used cultivation medium. However, recently, a defined cultivation medium, the so-called “Vienna Defined Medium” or “VD Medium”, was developed. By using this, typical shortcomings of complex media like batch-to-batch variability, as well as the occurrence of inhibiting compounds, can be eliminated, while average specific growth rate and final cell density of the model Archaeon Sulfolobus acidocaldarius match the Brock medium [15].
2. Cell Membrane Structure
Already, in 1973, the cell wall of S. acidocaldarius was isolated and investigated for its compounds and biochemical characteristics. These fundamental experiments showed the high robustness against the treatment with enzymes and reagents; in particular, ethylenediamine tetraacetic acid (EDTA), sodium dodecyl sulfate (SDS), dimethyl sulfoxide (DMSO) and Triton X-100. The absence of peptidoglycan in the membrane distinguishes Archaea from Bacteria [16].
In addition to the cell membrane, the cell envelope of Sulfolobus spp. consists of a proteinaceous layer called the surface (S-)layer. In Sulfolobus spp., the proteins SlaA and SlaB [17] were identified as the components of this layer, in controversy to many other Archaea’s S-layers consisting only of one protein. In Figure 1 a graphical illustration of the cell envelope is shown. SlaA serves as the sheath, whereas the SlaB protein makes up the shaft [17] and anchors the S-layer in the cytoplasmic membrane. The space in between, spanned by the proteins of the S-layer, represents the pseudoperiplasmic space [12].
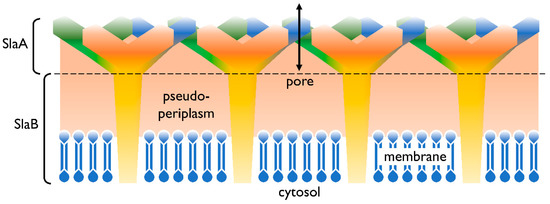
Figure 1. Cell envelope profile of Sulfolobus spp. The semi-permeable surface (S-)layer is comprised of the proteins SlaA (sheath) and SlaB (shaft). Both SlaA and SlaB are protein trimers and the contact area of these trimers is indicated with a dashed line. The salmon colored area represents the pseudoperiplasmic space. In Sulfolobus spp., the membrane is predominantly comprised of membrane spanning tetraether lipids [12][18].
The cell membrane, which encloses the cytoplasm, is composed of phospho- and glycolipids. A further crucial difference between archaeal and bacterial membrane lipids is the presence of ether bonds in the former instead of ester linkages. In most Archaea, a lipid bilayer comprised of glycerol diether lipids construct the cell membrane [12]. The core archaeal lipids are monomeric dialkyl glycerol diethers (commonly called archaeol or DGD). Their hydrophobic core of C20 isoprenoids is linked to the glycerol backbone at the sn-2,3 positions (Figure 2A). Two diether moieties can form glycerol dialkyl glycerol tetraether (called caldarchaeol or GDGT), by head to head linking (Figure 2B). The hydrophobic core is then comprised of C40 archaeal isoprenoids. Diether lipids form a bilayer in contrast to the monolayer formed by tetraether lipids. Both diether and tetraether structures exhibit variations in their polar head groups, cyclization and length of hydrophobic core (the latter is true for the archaeol of certain Archaea only). In Sulfolobus spp. the constituents of the cell membranes are predominantly tetraether lipids arranged as a monolayer. Furthermore, Sulfolobales’ membranes also contain calditol linked to the sn-1 position of the glycerol (Figure 2C and D). This is one of the major variants of caldarchaeol, and, based on early, inaccurate structure elucidation, is also known under the deceptive name glycerol dialkylnonitol tetraether (GDNT) [19][20][21][22][23].
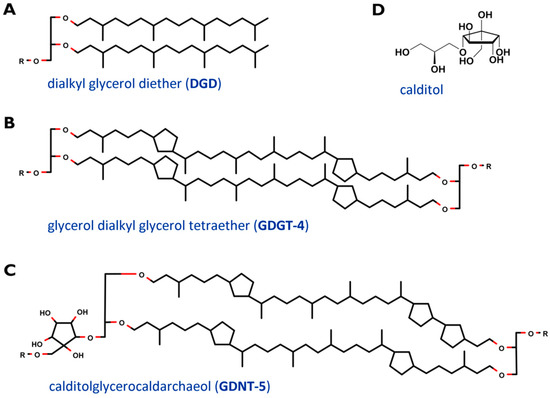
Figure 2. Core structure of major membrane lipids in Sulfolobus spp. (A) dialkyl glycerol diether (DGD); (B) glycerol dialkyl glycerol tetraether (GDGT); (C) glycerol dialkylnonitol tetraether (GDNT). R stands for polar head groups like (poly-) hexoses (Hex), inositolphosphate (IP), sulfonated trihexose (sulfono-Hex3), or can simply represent a single H- atom. The tetraether core structures are exemplarily depicted with 4 (GDGT-4) and 5 (GDNT-5) cyclopentane rings. (D) revised structure of the head group calditol, according to [22].
Table 1 gives an overview of the membrane lipid composition of the order Sulfolobales, in comparison to other well studied archaeal members. The halophilic membrane of Natronococcus is comprised of a mixture of C20-20 diether lipids (lipids harboring two alkyl chains in the lipid core, with 20 carbon atoms per chain) and C20-25 diether lipids (20 and 25 carbon atoms) [24], while Sulfolobales mainly consist of C40-40 tetraether lipids and partly of C20-20 diether lipids [25]. Methanogens like the Methanobacterium contain C20-20 diether lipids, as well as C40-40 tetraether lipids in varying ratios in their membrane [26]. GDNTs have only been found within the Crenarchaeota phylum so far [27].
Table 1. Overview membrane lipid composition of Sulfolobales, Natronococcus and Methanobacterium.
| Membrane Lipids | Sulfolobales | Natronococcus | Methanobacterium | Reference |
|---|---|---|---|---|
| C20-20 DGD | Σ15%–30% | Σ57%–77% | Σ12.8%–50% | [28][29][30][31][32] |
| DGD | ~5% | n.d. | + | [26][30][31] |
| IP-DGD | 10%-30% | n.d. | + | [26][30][31] |
| PG | - | 8–26% | - | [32] |
| PGP | - | 49–51% | - | [32] |
| PGP-Me | - | + | - | [33] |
| C20-25 DGD | - | Σ22%–44% | - | [32] |
| PG | - | 8%–9% | - | [32] |
| PGP | - | 14%–35% | - | [32] |
| C40-40 TEL (GDNT/GDGT) * | Σ70–85% | - | Σ50%–83% (GDGT only) | [28][29][30][31] |
| TEL | 7–9% | - | n.d. | [30][31] |
| Hex2-TEL | 57.8% | - | n.d. | [30][31] |
| IP-TEL | 3–36% | - | n.d. | [30][31] |
| Sulfono-Hex3-TEL-IP | 1–11% | - | n.d. | [30][31] |
| Hex-TEL-IP | + | - | n.d. | [25] |
| Hex2-TEL-IP | 4%–82% | - | n.d. | [30][31] |
| C40-40 GDNT | Σ 68–80% | - | - | [21][34][35][36][37] |
| Hex-GDNT | + | - | - | [34][35] |
| IP-GDNT | + | - | - | [34][35] |
3. Biosynthesis of Archaeal Membrane Lipids
The biosynthesis of membrane lipids is different between the tree domains of life, as well as within one domain. In general, archaeal membrane lipids are formed by multiple consecutive enzymatic steps (Figure 3), starting with the synthesis of the isoprenoid building blocks isopentyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). This is done via the MEP/DOXP pathway, the classical mevalonate (MVA) or the alternate MVA pathway. The alternate MVA pathway is most common in Archaea, with the exception of Sulfolobales that utilize the classical MVA pathway [38]. According to Boucher et al., Sulfolobus acquired the enzymes of the classical MVA pathway through lateral gene transfer from Eukaryotes [39]. Furthermore, geranyl diphosphate (GPP) is formed through condensation reactions of DMAPP and IPP. The carbon 10 compound is then condensed with several IPP molecules, to form farnesyl (C15), geranylgeranyl (C20) or farnesylgeranyl (C25) diphosphate [40]. For the formation of the characteristic ether bonded geranylgeranylglyceryl diphosphate (GGGP), geranylgeranyl diphosphate (GGPP) of the pathway described before is needed, as well as glycerol-1-phosphate. The latter is formed through the reduction of dihydroxyacetone phosphate (DHAP), utilizing NADH. The diether DGGGP (2,3-O-geranylgeranylglyceryl diphosphate) is formed by GGGP and GGPP. With this step, the core lipid is formed and is activated by cytidine triphosphate (CTP) in the following. This is done by the CDP archaeol synthase [38][41].
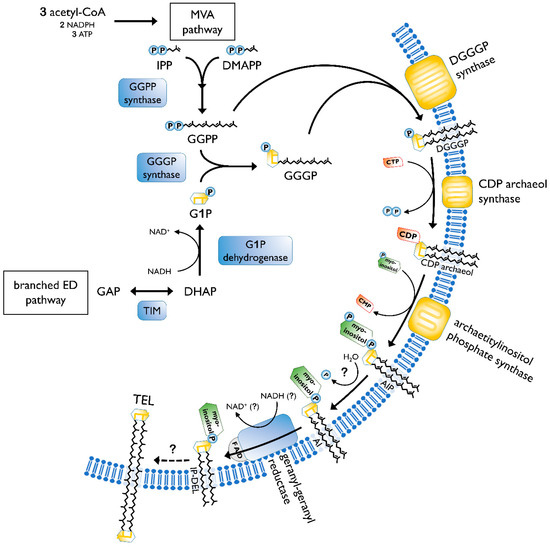
Figure 3. Pathway of archaeal lipid biosynthesis. MVA, mevalonate pathway; IPP, isopentyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; GGPP, geranylgeranyl diphosphate; ED pathway, Entner–Doudoroff pathway; GAP, glyceraldehyde 3-phosphate; TIM, triosephosphate isomerase; DHAP, dihydroxyacetone phosphate; G1P, glycerol-1-phosphate; GGGP, geranylgeranylglyceryl phosphate; DGGGP, 2,3-O-geranylgeranylglyceryl diphosphate; CTP, cytidine triphosphate; CMP, cytidine monophosphate; CDP, cytidine diphosphate; AIP archaetidylinositol phosphate; AI, archaetidylinositol; IP-DEL inositolphosphate diether lipid; TEL, tetraether lipid; P, phosphate; PP, pyrophosphate. A description of the biosynthesis steps is given in the text. Figure based on [38][42].
Next, the reactive cytidine diphosphate (CDP) head group is replaced by polar head groups, such as serine, ethanolamin, glycerol or myo-inositol. Then, geranylgeranyl reductases catalyze the hydrogenation of the unsaturated DGGGP and hence completing the structure of the archaeol [38]. It is still not clear at which step the saturation occurs. However, Koga and Morii (2007) proposed that it took place after the addition of the polar head group [40].
The caldarchaeol, which represents the characteristic tetraether lipid structure, is formed from two archaeols undergoing a head to head condensation. This was proven by labeling experiments in Thermoplasma acidophilum [38]. Until now, the enzyme catalyzing the tetraether bond has not been identified. Additionally, the biosynthesis of cyclopentane rings is also only partially known. A hypothesis about tetraether and cyclopentane ring formation was offered by Villanueva et al. They suggested a “multiple-key, multiple-lock” mechanism and proposed that the cyclopentane rings are being formed before the tetraethers configuration. The coupling of two diethers is hypothetically realized by phytoene synthase, which has been elucidated in the archaeal genome. However, this theory still has to be verified by experiments [43]. A strong argument against this hypothesis is that the ring index (RI) quickly changes upon changed environmental conditions [34]. Following the hypothesis from Villanueva, a change in the ring number would require a complete cycle of lipid degradation and synthesis of the new lipids with different ring numbers. The findings by Zeng et al. [44] of an ordered process of cyclization counters the proposition that the rings are already existent in the isoprenoids when added to the glycerol backbone. It confirms a common hypothesis that the cyclization occurs within the GDGT chain. Albeit, it is still not certain if the saturation of the chain, as well as the different head groups, plays a role in the ring formation process. Two genes, Saci_1585 and Saci_0240, were determined to be coding for GDGT ring synthases (Grs). Both respective proteins, GrsA and GrsB, belong to the S-adenosylmethionine (SAM) protein family [44]. Members of the same family are also responsible for the formation of the calditol moiety [27]. Hence, both incorporations require a free radical mechanism. GrsA is responsible for the introduction of rings at the C7 position of the core GDGT lipid. This has to be done prior to the cyclization of the C3 position carried out by GrsB [44]. Further, two other genes, Saci_0421 and Saci_1201, in S. acidocaldarius were identified for influencing the number of cyclopentane rings. If deleted, the number decreases. However, their exact function in the cyclization process has not yet been determined [45].
Additional to the typical caldarchaeol, the membranes of Sulfolobus also exhibit GDNT. One glycerol is replaced by a cyclopentane containing moiety, called calditol (Figure 2D). For its biosynthesis, D-glucose is converted into calditol via an “inositol-like” pathway [46]. C-C bond is formed between C1 and C5 of glucose. The possible mechanism of calditol formation includes starting the oxidation at C4, which resembles the formation of myo-inositol. Both moieties are present as polar groups in the cell membrane of Sulfolobales [47].
A connection between the lipid synthesis and homeoviscous adaption is certainly present and of crucial importance. However, on a genetic, regulatory level, this influence on the lipid biosynthesis in response to environmental changes remains an untouched field of investigation and represents a promising area for future research.
4. Effect of Cyclopentane Rings, DEL:TEL Ratio and Length of Carbon Chain on the Archaeal Membrane
The effects of cyclopentane rings on the properties of archaeal membranes is usually studied in vitro, by investigating liposomes formed of the respective diether lipids (DEL) and tetraether lipids (TEL).
TELs, the main constituents of crenarchaeal membranes, can exhibit either GDGT or GDNT as hydrophobic core. Up to eight cyclopentane rings can be incorporated within this core [48][49]. Differential scanning calorimetry and perturbation calorimetry were used to determine the effect of the incorporation of cyclopentane rings. Findings in liposomes made with extracted GDGT and GDNT lipids of S. acidocaldarius suggest that a higher number of cyclopentane rings in the hydrophobic core affects the membrane packing and increases the transition temperature. Albeit, the volume of the membrane is relatively constant between liposomes containing low and high amounts of cyclopentane rings [50]. In general, the compressibility, the relative change of volume in response to a change in environment conditions [51], of tetraether liposomes seems to be lower when compared to diester liposomes, meaning that TEL liposomes are less influenced by stressors such as temperature [52]. These experimental findings are backed by in silico results. Molecular modeling proposed that the incorporation of cyclopentane rings results in a tighter and more rigid membrane [53]. However, it was shown that the isothermal compressibility values increase with the number of cyclopentane rings in a nonlinear manner. The most tightly packed membrane was not observed at the highest number of rings, but at the optimal growth temperature of the organism [50]. These results indicate that cyclopentane rings might not be the only aspect affecting the rigidity and packing of archaeal membranes [50][53][54].
Diether lipids, like diester lipids, form bilayers, while tetraether lipids can span the lamellar structure, and thus, form monolayers [55]. Archaeosomes constructed from total polar lipid extracts of methanogens and halophiles, mainly consisting of diether lipids, are less stable against extreme pH treatment, measured by entrapped radioactive compounds, when compared to archaeosomes created from lipids extract of T. acidophilum. The incorporation of TEL increases the stability towards high temperature, pH treatment and serum proteins [56]. Liposomes with a high TEL content could even endure sterilization (121 °C, 1.034 bar, 15 min). There is a clear relationship between the leakage rate of a fluorescent dye and TEL presence in the membrane. Albeit, archaeosomes containing TEL or DEL can endure enzymatic treatment with pancreatic lipase [57][58]. In general, the increase of TEL content in the membrane aids the flux regulation of solutes and protons [59].
The chain-length variation of the hydrophobic lipid core is typical for Bacteria and Eukaryotes as a response to environmental stress [60]. Hereby, the difference between altering the length of hydrocarbon chains and changing from DEL to TEL has to be distinguished. While switching from diether to tetraether is a process in which two diethers are conjoined together, Bacteria alter their length of fatty acid chains; e.g., switching from C16 to C18 [61]. Membranes of alkaliphilic Archaea are composed of a mixture of C20-20 and C20-25 diether lipids, resulting in higher permeability and fluidity at low temperature. However, establishing a connection between this ratio and the framework homeoviscous adaption has not been possible yet [59][62].
In addition to high temperature adaption, methanogens, like Methanococcoides burtonii, can adapt to low temperature by unsaturation, forming double bonds in the hydrocarbon chain [63], a feature that appears to be absent entirely in Sulfolobales.
5. Membrane Regulators
Three primary functions have been attributed to biological membranes. First, they prevent the entrance of toxic substances into the cell. Secondly, they provide a framework for the channels and receptors responsible for the inward and outward transport of nutrients, ions and waste products. Thirdly, they provide separate environments between organelles. Membrane regulators, such as sterols, have the ability to regulate the membrane dynamics in Bacteria and Eukarya. They are known to protect the membrane from amphipathic toxins and stabilize its structure [64].
In a current review, Salvador-Castell et al. suggested possible members of archaeal membrane regulators. Polyterpenes, like carotenoids, polyprenols, quinones and apolar polyisoprenoids are likely to function as those, and help the cell membrane to adapt to physiological and physicochemical stressors. Sulfolobales feature all four of the proposed membrane regulators. As for carotenoids, β-carotene and zeaxanthin have been shown to be present in Sulfolobales. In Bacteria and Eukaryotes this said polyterpene has an antioxidant effect and intervenes with the membrane’s physicochemical properties. However, the function in Archaea is still unknown, together with the insertion process into the monolayer membrane of Sulfolobus spp. Polyprenols are involved in membrane protein glycosylation and protect cells against free radicals. Its length of side chains and number of unsaturations in polyphenyl-alcohol were associated with environmental conditions such as temperature. Although quinones have not been found in all Archaea, sulfolobusquinones are present in Sulfolobales. Besides its involvement in the transfer of electrons and protons in the respiratory chain, this potential archaeal membrane regulator is engaged in membrane modulation and stabilization. In Saccharolobus solfataricus (previously Sulfolobus solfataricus), the quinone side chains are affected by oxygen availability and carbon source. Apolar polyisoprenoids’ function is still being investigated. Longer apolar isoprenoids are present in hyperthermophilic Thermococcales, as well as extreme halophiles such as Haloferacales and Halobacteriales, while thermoacidphilic Sulfolobales synthesize mainly short ones. This can be due to the fact that long-chain polyisoprenoids are correlated to bilayer and not monolayer, which Sulfolobus membrane is mainly comprised of due to the high tetraether lipid content. In the bilayer, this polyterpene acts as a membrane stabilizer towards stress factors [65].
This entry is adapted from the peer-reviewed paper 10.3390/ijms21113935
References
- Quehenberger, J.; Shen, L.; Albers, S.-V.; Siebers, B.; Spadiut, O. Sulfolobus—A Potential Key Organism in Future Biotechnology. Front Microbiol. 2017, 8, 2474.
- Dumorné, K.; Córdova, D.C.; Astorga-Eló, M.; Renganathan, P. Extremozymes: A Potential Source for Industrial Applications. J. Microbiol. Biotechnol. 2017, 27, 649–659.
- Nicolaus, B.; Gambacorta, A.; Basso, A.L.; Riccio, R.; De Rosa, M.; Grant, W.D. Trehalose in Archaebacteria. Syst. Appl. Microbiol. 1988, 10, 215–217.
- Charlesworth, J.; Burns, B. Untapped Resources: Biotechnological Potential of Peptides and Secondary Metabolites in Archaea. Archaea 2015, 2015, 1–7.
- Patel, G.B.; Sprott, G.D. Archaeobacterial Ether Lipid Liposomes (Archaeosomes) as Novel Vaccine and Drug Delivery Systems. Crit. Rev. Biotechnol. 1999, 19, 317–357.
- Peng, N.; Han, W.; Li, Y.; Liang, Y.; She, Q. Genetic technologies for extremely thermophilic microorganisms of Sulfolobus, the only genetically tractable genus of crenarchaea. Sci. Chin. Life Sci. 2017, 60, 370–385.
- Grogan, D.W. Phenotypic characterization of the archaebacterial genus Sulfolobus: Comparison of five wild-type strains. J. Bacteriol. 1989, 171, 6710–6719.
- Van de Vossenberg, J.L.C.M.; Driessen, A.J.M.; Konings, W.N. The essence of being extremophilic: The role of the unique archaeal membrane lipids. Extremophiles 1998, 2, 163–170.
- Chen, L.; Brügger, K.; Skovgaard, M.; Redder, P.; She, Q.; Torarinsson, E.; Greve, B.; Awayez, M.; Zibat, A.; Klenk, H.-P.; et al. The Genome of Sulfolobus acidocaldarius, a Model Organism of the Crenarchaeota. J. Bacteriol. 2005, 187, 4992–4999.
- O’Connor, E.M.; Shand, R.F. Halocins and sulfolobicins: The emerging story of archaeal protein and peptide antibiotics. J. Ind. Microbiol. Biotechnol. 2002, 28, 23–31.
- Guo, L.; Brügger, K.; Liu, C.; Shah, S.A.; Zheng, H.; Zhu, Y.; Wang, S.; Lillestøl, R.K.; Chen, L.; Frank, J.; et al. Genome Analyses of Icelandic Strains of Sulfolobus islandicus, Model Organisms for Genetic and Virus-Host Interaction Studies. J. Bacteriol. 2011, 193, 1672–1680.
- Albers, S.-V.; Meyer, B. The archaeal cell envelope. Nat. Rev. Microbiol. 2011, 9, 414–426.
- Albers, S.-V.; Jarrell, K.F. The archaellum: How Archaea swim. Front. Microbiol. 2015, 6.
- Glansdorff, N. Extremophiles—Volume I; EOLSS Publications: Abu Dhabi, UAE, 2009; ISBN 978-1-905839-93-3.
- Quehenberger, J.; Albersmeier, A.; Glatzel, H.; Hackl, M.; Kalinowski, J.; Spadiut, O. A defined cultivation medium for Sulfolobus acidocaldarius. J. Biotechnol. 2019, 301, 56–67.
- Weiss, R. Subunit Cell Wall of Sulfolobus acidocaldarius. J. Bacteriol. 1974, 118, 275–284.
- Veith, A.; Klingl, A.; Zolghadr, B.; Lauber, K.; Mentele, R.; Lottspeich, F.; Rachel, R.; Albers, S.-V.; Kletzin, A. Acidianus, Sulfolobus and Metallosphaera surface layers: Structure, composition and gene expression. Mol. Microbiol. 2009, 73, 58–72.
- Gambelli, L.; Meyer, B.H.; McLaren, M.; Sanders, K.; Quax, T.E.F.; Gold, V.A.M.; Albers, S.-V.; Daum, B. Architecture and modular assembly of Sulfolobus S-layers revealed by electron cryotomography. Proc. Natl. Acad. Sci. USA 2019, 116, 25278–25286.
- Kate, M. Chapter 9 Membrane lipids of archaea. In New Comprehensive Biochemistry; Kates, M., Kushner, D.J., Matheson, A.T., Eds.; Elsevier: New York, NY, USA, 1993; Volume 26, pp. 261–295.
- Klingl, A. S-layer and cytoplasmic membrane—exceptions from the typical archaeal cell wall with a focus on double membranes. Front. Microbiol. 2014, 5.
- Sugai, A.; Sakuma, R.; Fukuda, I.; Kurosawa, N.; Itoh, Y.H.; Kon, K.; Ando, S.; Itoh, T. The structure of the core polyol of the ether lipids from Sulfolobus acidocaldarius. Lipids 1995, 30, 339–344.
- Blériot, Y.; Untersteller, E.; Fritz, B.; Sinaÿ, P. Total Synthesis of Calditol: Structural Clarification of this Typical Component of Archaea Order Sulfolobales. Chem.—Eur. J. 2002, 8, 240–246.
- De Rosa, M.; De Rosa, S.; Gambacorta, A.; Bu’Lockt, J.D. Structure of calditol, a new branched-chain nonitol, and of the derived tetraether lipids in thermoacidophile archaebacteria of the Caldariella group. Phytochemistry 1980, 19, 249–254.
- Gibson, J.A.E.; Miller, M.R.; Davies, N.W.; Neill, G.P.; Nichols, D.S.; Volkman, J.K. Unsaturated diether lipids in the psychrotrophic archaeon Halorubrum lacusprofundi. Syst. Appl. Microbiol. 2005, 28, 19–26.
- Jensen, S.M.; Brandl, M.; Treusch, A.H.; Ejsing, C.S. Structural characterization of ether lipids from the archaeon Sulfolobus islandicus by high-resolution shotgun lipidomics. J. Mass Spectrom. 2015, 50, 476–487.
- Koga, Y.; Nishihara, M.; Morii, H.; Akagawa-Matsushita, M. Ether polar lipids of methanogenic bacteria: Structures, comparative aspects, and biosyntheses. Microbiol. Rev. 1993, 57, 164–182.
- Zeng, Z.; Liu, X.-L.; Wei, J.H.; Summons, R.E.; Welander, P.V. Calditol-linked membrane lipids are required for acid tolerance in Sulfolobus acidocaldarius. Proc. Natl. Acad. Sci. USA 2018, 115, 12932–12937.
- Morii, H.; Koga, Y. Tetraether type polar lipids increase after logarithmic growth phase of Methanobacterium thermoautotrophicum in compensation for the decrease of diether lipids. FEMS Microbiol. Lett. 1993, 109, 283–287.
- Tornabene, T.G.; Langworthy, T.A. Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science 1979, 203, 51–53.
- Jensen, S.M.; Neesgaard, V.L.; Skjoldbjerg, S.L.N.; Brandl, M.; Ejsing, C.S.; Treusch, A.H. The Effects of Temperature and Growth Phase on the Lipidomes of Sulfolobus islandicus and Sulfolobus tokodaii. Life 2015, 5, 1539–1566.
- Quehenberger, J.; Pittenauer, E.; Allmaier, G.; Spadiut, O. The influence of the specific growth rate on the lipid composition of Sulfolobus acidocaldarius. Extremophiles 2020.
- Nicolaus, B.; Lanzotti, V.; Trincone, A.; De Rosa, M.; Grant, W.D.; Gambacorta, A. Glycine betaine and polar lipid composition in halophilic archaebacteria in response to growth in different salt concentrations. FEMS Microbiol. Lett. 1989, 59, 157–160.
- Angelini, R.; Corral, P.; Lopalco, P.; Ventosa, A.; Corcelli, A. Novel ether lipid cardiolipins in archaeal membranes of extreme haloalkaliphiles. Biochim. Biophys. Acta 2012, 1818, 1365–1373.
- De Rosa, M.; Esposito, E.; Gambacorta, A.; Nicolaus, B.; Bu’Lock, J.D. Effects of Temperature on ether lipid composition of Caldariella acidophila. Phytochemistry 1980, 19, 827–831.
- Bode, M.L.; Buddoo, S.R.; Minnaar, S.H.; du Plessis, C.A. Extraction, isolation and NMR data of the tetraether lipid calditoglycerocaldarchaeol (GDNT) from Sulfolobus metallicus harvested from a bioleaching reactor. Chem. Phys. Lipids 2008, 154, 94–104.
- Rosa, M.D.; Gambacorta, A.; Nicolaus, B.; Chappe, B.; Albrecht, P. Isoprenoid ethers; backbone of complex lipids of the archaebacterium Sulfolobus solfataricus. Biochim. Biophys. Acta (BBA) Metab. 1983, 753, 249–256.
- Gliozzi, A.; Paoli, G.; De Rosa, M.; Gambacorta, A. Effect of isoprenoid cyclization on the transition temperature of lipids in thermophilic archaebacteria. Biochim. Biophys. Acta (BBA)—Biomembr. 1983, 735, 234–242.
- Jain, S.; Caforio, A.; Driessen, A.J.M. Biosynthesis of archaeal membrane ether lipids. Front. Microbiol. 2014, 5, 641.
- Boucher, Y.; Kamekura, M.; Doolittle, W.F. Origins and evolution of isoprenoid lipid biosynthesis in archaea. Mol. Microbiol. 2004, 52, 515–527.
- Koga, Y.; Morii, H. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol. Mol. Biol. Rev. 2007, 71, 97–120.
- Koga, Y. Biosynthesis and evolution of archaeal membranes and ether phospholipids. In Biogenesis of Fatty Acids, Lipids and Membranes; Geiger, O., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 539–552. ISBN 978-3-319-50430-8.
- Morii, H.; Kiyonari, S.; Ishino, Y.; Koga, Y. A novel biosynthetic pathway of archaetidyl-myo-inositol via archaetidyl-myo-inositol phosphate from CDP-archaeol and D-glucose 6-phosphate in methanoarchaeon Methanothermobacter thermautotrophicus cells. J. Biol. Chem. 2009, 284, 30766–30774.
- Villanueva, L.; Damsté, J.S.S.; Schouten, S. A re-evaluation of the archaeal membrane lipid biosynthetic pathway. Nat. Rev. Microbiol. 2014, 12, 438–448.
- Zeng, Z.; Liu, X.-L.; Farley, K.R.; Wei, J.H.; Metcalf, W.W.; Summons, R.E.; Welander, P.V. GDGT cyclization proteins identify the dominant archaeal sources of tetraether lipids in the ocean. Proc. Nat. Acad. Sci. USA 2019, 116.
- Guan, Z.; Delago, A.; Nußbaum, P.; Meyer, B.; Albers, S.-V.; Eichler, J. Gene deletions leading to a reduction in the number of cyclopentane rings in Sulfolobus acidocaldarius tetraether lipids. FEMS Microbiol. Lett. 2018, 365.
- Gambacorta, A.; Caracciolo, G.; Trabasso, D.; Izzo, I.; Spinella, A.; Sodano, G. Biosynthesis of calditol, the cyclopentanoid containing moiety of the membrane lipids of the archaeon Sulfolobus solfataricus. Tetrahedron Lett. 2002, 43, 451–453.
- Yamauchi, N.; Ueoka, H.; Kamada, N.; Murae, T. Resemblance of Carbocycle Formation from Carbohydrates between Archaea and Eucarya/Eubacteria. Biosynthesis of Calditol, the Characteristic Lipid-Content Molecule in Sulfolobus acidocaldarius. Bull. Chem. Soc. Jpn. 2004, 77, 771–778.
- Macalady, J.L.; Vestling, M.M.; Baumler, D.; Boekelheide, N.; Kaspar, C.W.; Banfield, J.F. Tetraether-linked membrane monolayers in Ferroplasma spp: A key to survival in acid. Extremophiles 2004, 8, 411–419.
- Schouten, S.; Hopmans, E.C.; Sinninghe Damsté, J.S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review. Org. Geochem. 2013, 54, 19–61.
- Zhai, Y.; Lee-Gau Chong, P.; Taylor, L.J.-A.; Erlkamp, M.; Grobelny, S.; Czeslik, C.; Watkins, E.; Winter, R. Physical Properties of Archaeal Tetraether Lipid Membranes As Revealed by Differential Scanning and Pressure Perturbation Calorimetry, Molecular Acoustics, and Neutron Reflectometry: Effects of Pressure and Cell Growth Temperature. Langmuir 2012, 28, 5211–5217.
- Heremans, K. Chapter 1—Pressure—Temperature effects on protein conformational states. In Chemistry at Extreme Conditions; Manaa, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–27. ISBN 978-0-444-51766-1.
- Chong, P.L.-G.; Sulc, M.; Winter, R. Compressibilities and Volume Fluctuations of Archaeal Tetraether Liposomes. Biophys. J. 2010, 99, 3319–3326.
- Gabriel, J.L.; Chong, P.L.-G. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem. Phys. Lipids 2000, 105, 193–200.
- Chong, P.L.-G.; Ayesa, U.; Prakash Daswani, V.; Hur, E.C. On physical properties of tetraether lipid membranes: Effects of cyclopentane rings. Archaea 2012, 2012.
- Chattopadhyay, A. Membrane Organization and Dynamics; Springer: Berlin, Germany, 2017; ISBN 978-3-319-66601-3.
- Choquet, C.G.; Patel, G.B.; Sprott, G.D.; Beveridge, T.J. Stability of pressure-extruded liposomes made from archaeobacterial ether lipids. Appl. Microbiol. Biotechnol. 1994, 42, 375–384.
- Choquet, C.G.; Patel, G.B.; Sprott, G.D. Heat sterilization of archaeal liposomes. Can. J. Microbiol. 1996, 42, 183–186.
- Patel, G.B.; Agnew, B.J.; Deschatelets, L.; Fleming, L.P.; Sprott, G.D. In vitro assessment of archaeosome stability for developing oral delivery systems. Int. J. Pharm. 2000, 194, 39–49.
- Oger, P.M.; Cario, A. Adaptation of the membrane in Archaea. Biophys. Chem. 2013, 183, 42–56.
- Zhang, Y.-M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233.
- Saunders, L.P.; Sen, S.; Wilkinson, B.J.; Gatto, C. Insights into the Mechanism of Homeoviscous Adaptation to Low Temperature in Branched-Chain Fatty Acid-Containing Bacteria through Modeling FabH Kinetics from the Foodborne Pathogen Listeria monocytogenes. Front. Microbiol. 2016, 7.
- Akasaka, K.; Matsuki, H. High Pressure Bioscience: Basic Concepts, Applications and Frontiers; Springer: Berlin, Germany, 2015; ISBN 978-94-017-9918-8.
- Nichols, D.S.; Miller, M.R.; Davies, N.W.; Goodchild, A.; Raftery, M.; Cavicchioli, R. Cold Adaptation in the Antarctic Archaeon Methanococcoides burtonii Involves Membrane Lipid Unsaturation. J. Bacteriol. 2004, 186, 8508–8515.
- Dufourc, E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008, 1, 63–77.
- Salvador-Castell, M.; Tourte, M.; Oger, P.M. In Search for the Membrane Regulators of Archaea. Int. J. Mol. Sci. 2019, 20, 4434.
