4.1. Substrate
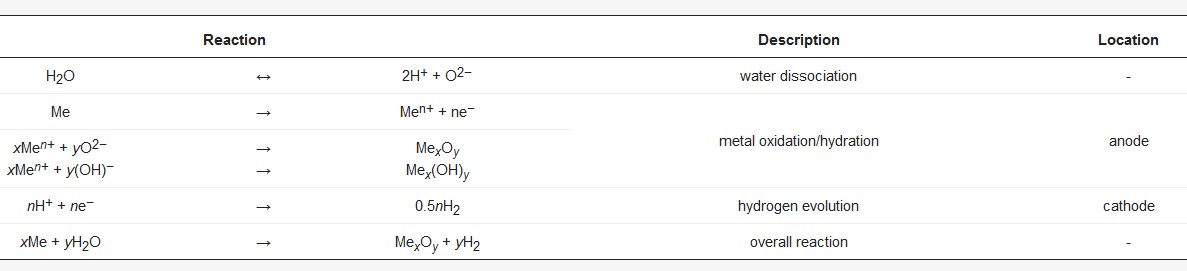
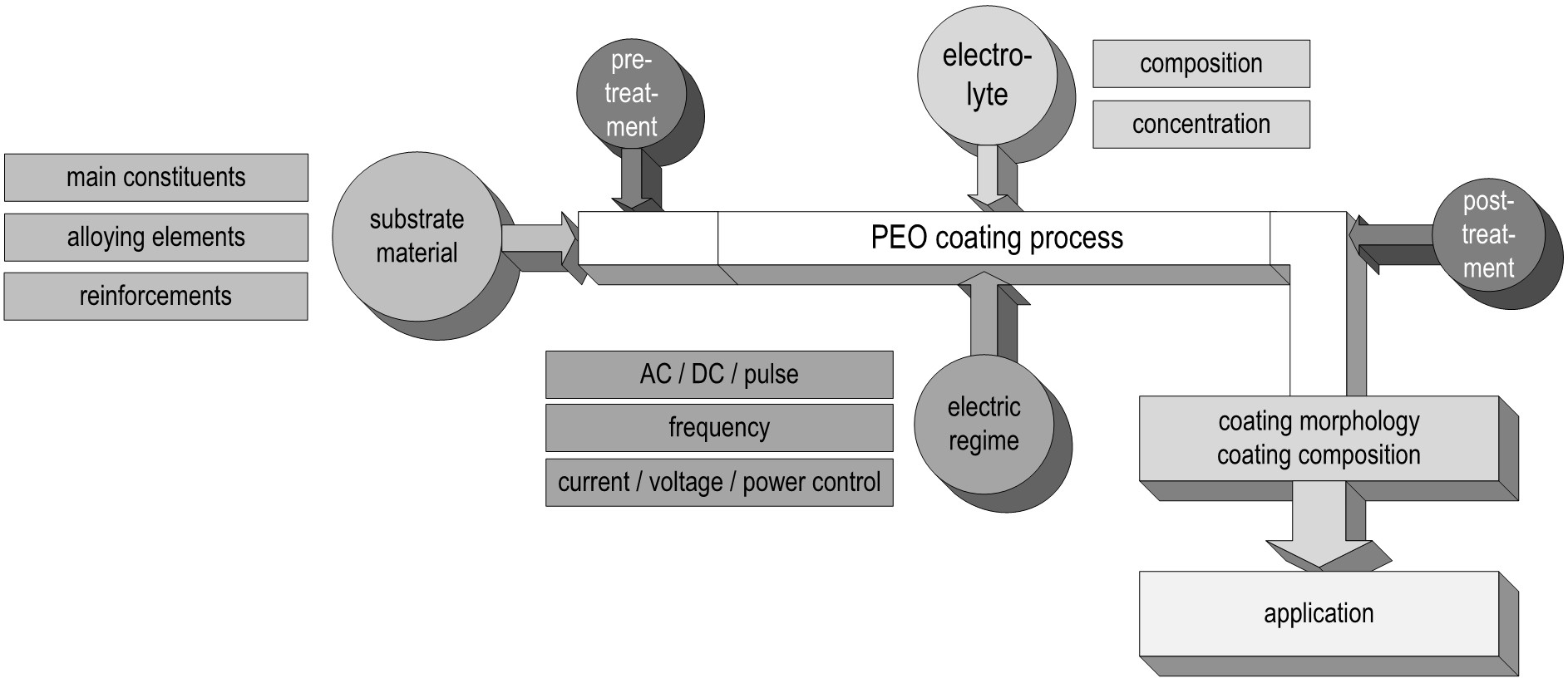
Plasma electrolytic oxidation in common low concentration aqueous electrolytes is at its core a conversion coating process. Hence, the nature of the oxide strongly depends on the substrate composition. The metal ions participating in the electrochemical reactions during the PEO process () are determined by the treated material. Generally, oxides of the substrate metal are the main constituents of the coatings. The substrate conversion naturally includes alloying elements and precipitates in the metal as well as reinforcement phases in case of metal matrix composites. For aluminum, this is shown in and . shows a scanning electron microscopy (SEM) picture of the oxide/substrate interface in the cross section of an oxide coating, produced by PEO in an alkaline silicate electrolyte on thermally-sprayed aluminum comprised of copper particles in BSE-mode (element contrast).
Figure 4. Cross section of the oxide/substrate interface of a PEO coating produced on thermally sprayed aluminum comprised of copper particles (P – PEO layer, S – Substrate). The brighter regions reflect a higher atomic number of the displayed material (SEM in BSE-mode – element contrast).
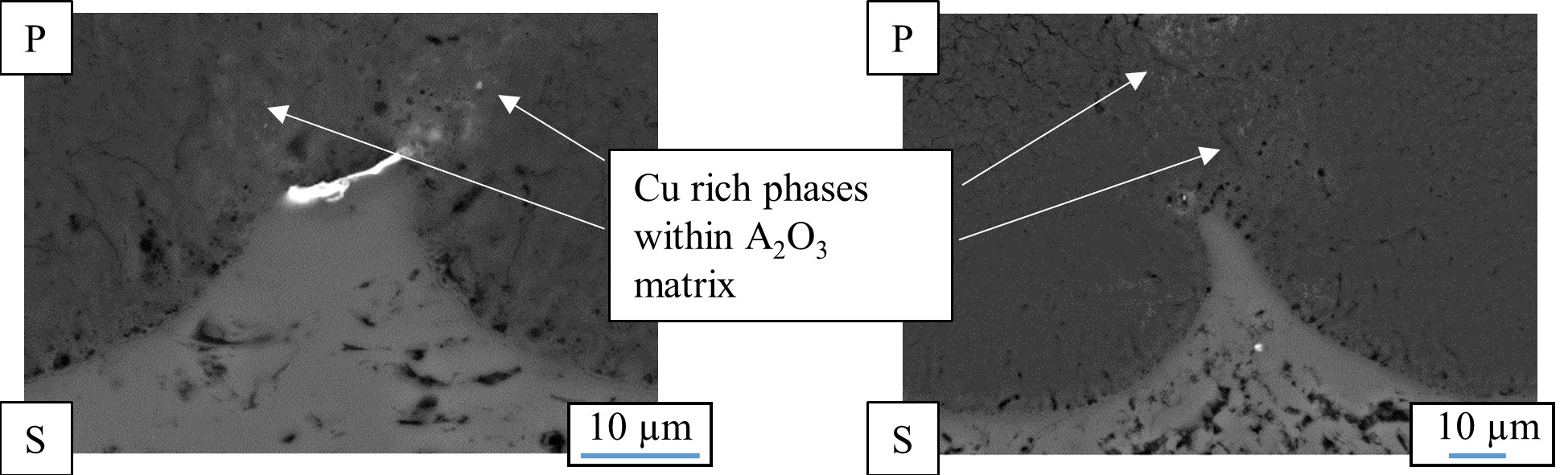
Figure 5. Cross section of the oxide produced in a PEO process on a SiC- (
a) and a Al
2O
3 - (
b) particle- reinforced aluminum alloy of the 2000 series [
57]. (Reprinted from [
57]. Copyright from 2016 IOP).
The bright fraction in the image represents a copper particle in the substrate. In the oxide above, the copper is obviously incorporated. It is also noticeable that the copper particle obstructs the conversion of the substrate, as in the surroundings, more of the substrate material has been consumed by the conversion coating process. shows a cross section of an oxide coating produced on an aluminum matrix composite (AMC) reinforced with silicon carbide or aluminum oxide particles.
It is obvious that the presence of SiC in the aluminum matrix leads to an increased number of flaws in the oxide, which are correlated to gas evolution at the electrically conductive particles. Remains of the particles, which are also partly converted, can be found in the pores of the coatings. However, under the high over-potential, the conversion of the silicon carbide particles takes place, presumably under gas evolution. This also results in a lower coating thickness, since the current efficiency is deteriorated by the evolution of gas. Unlike the presence of SiC, the presence of electrically non-conductive Al
2O
3 particles within the substrate does not affect the morphology or mechanical properties of the resulting PEO layer [
57].
For aluminum in general, the existence of alloying elements like zinc, copper, or magnesium is likely to impede the transition from the metastable γ-phase to the high-temperature α-phase [
58,
59]. In addition, the alloying elements significantly influence the oxide phase distribution within the PEO layer [
18]. Meanwhile, for magnesium alloys, the achievable coating thickness grows with an increasing content of aluminum or rare earths [
55].
However, the coating composition and morphology are, not only influenced by the substrate material, but can also be altered by the incorporation of electrolyte constituents, as will be shown in the following section.
4.2. Electrolyte
Section 3 includes a classification of electrolyte components with regard to their passivating or dissolving behavior towards the substrate. Nevertheless, other classifications are possible. With regard to the incorporation of foreign compounds into the oxide coating, electrolytes are classified as follows: (1) electrolytes leading only to oxygen incorporation, (2) electrolytes leading to the incorporation of foreign compounds by anions, (3) electrolytes leading to the incorporation of foreign compounds by cations, and (4) electrolytes containing macroscopic particles, which are incorporated into the oxide by cataphoretic processes [
17].
Common salts for the PEO of aluminum, magnesium, titanium, and their alloys in alkaline media are, amongst others, silicates, phosphates, aluminates, fluorides, borates, and stannates. Especially for the PEO of magnesium and its alloys, acidic or pH-neutral electrolyte compositions are used instead of alkaline electrolytes, e.g., fluoric acid, phosphoric acid, and/or boric acid in combination with organic additives, c.f. [
60,
61]. By incorporating elements provided by the electrolyte, the composition of the oxide can be altered substantially.
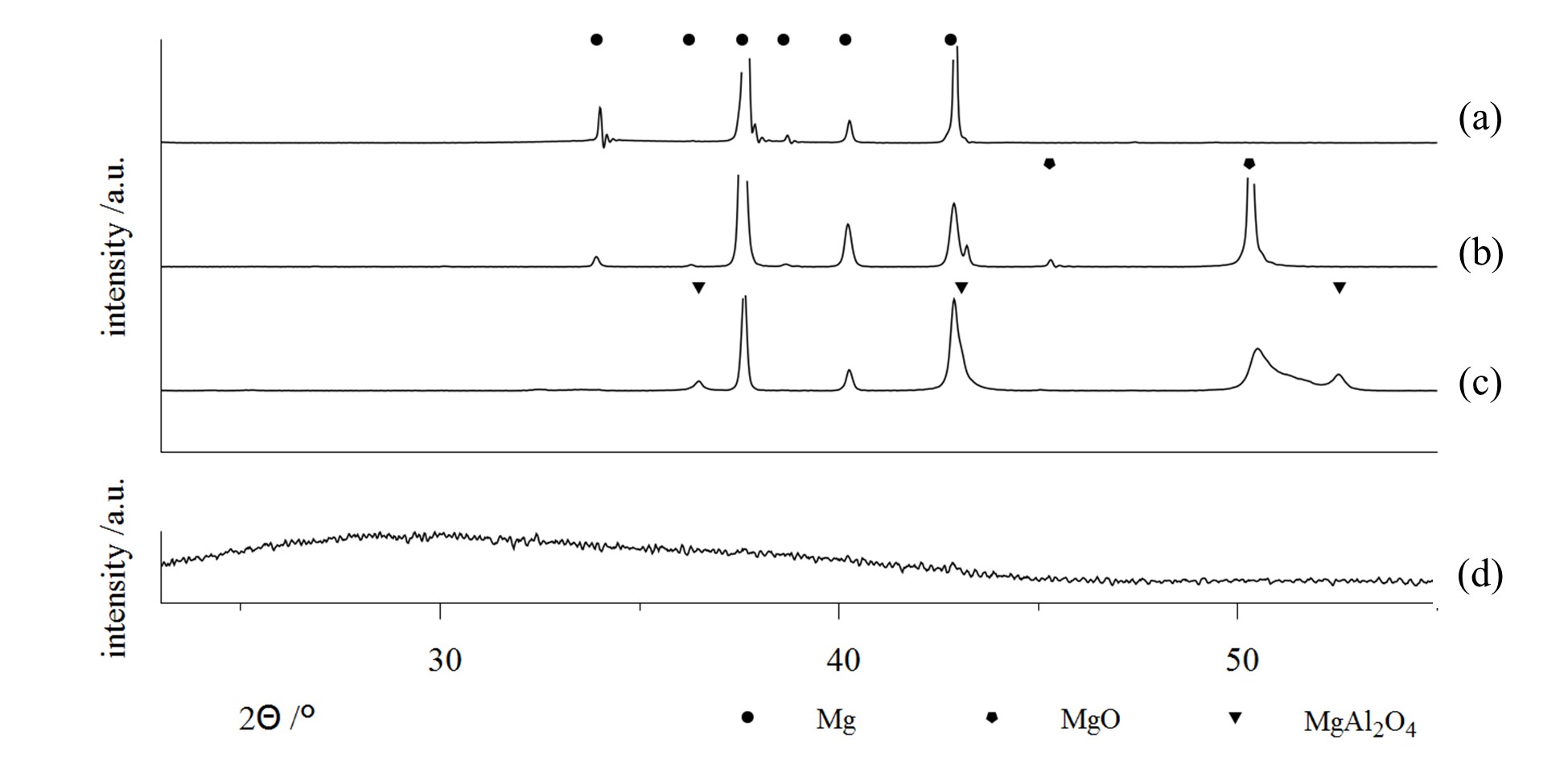
Some examples are shown in , which contains x-ray diffractograms of uncoated Mg-AZ31 (a) as well as samples of the same material, which were treated by PEO within different alkaline media (b–d). The use of a low concentration phosphate electrolyte leads to the formation of a magnesia layer by substrate conversion. On the other side, highly concentrated solutions allow for the incorporation of electrolyte constituents into the resulting layer. In this way, PEO layers are formed in aluminate-rich solutions, which contain high proportions of crystalline MgAl
2O
4 spinels (6c) [
21]. By using silicate-rich electrolytes, it is even possible to completely replace MgO in the PEO layer and to produce coatings, consisting of amorphous Mg / Si mixed oxides (6d), e.g., [
20]. Since aluminum- or silicon-containing mixed oxides are usually superior over magnesia or titania in regards to their hardness and chemical resistivity, this approach is relevant for the PEO of magnesium and titanium alloys. Therefore, the method is converted from a conversion process to a mixed form of conversion and deposition. Another prominent example of the incorporation of electrolyte constituents into the oxide coatings is the formation of hydroxyapatite-containing PEO coatings on titanium from calcium- and phosphorus-containing electrolytes, c.f. [
43,
44]. Additionally, the coloring of working pieces by PEO for decorative or optical applications (e.g., blackened by use of vanadate ions) is already in practical use, c.f. [
34,
36].
Figure 6. X-ray Diffraction (XRD)-diffractograms on bare AZ31 substrate, (a) and PEO coatings on AZ31 generated within several alkaline electrolytes; a low concentration phosphate solution, designed for substrate conversion, (b) high concentration silicate and aluminate electrolytes, designed for focused incorporation of electrolyte constituents (c,d) [
20,
21] (Apapted from [
20,
21]).
For the PEO of aluminum, the widely-used silicate components lead to the incorporation of large amounts of silicon oxides or alumina-silica-mixed-phases (e.g., mullite), have a passivating effect on the aluminum substrate, while also not dissolving the formed alumina. This generally increases the achievable coating thickness and results in a compact morphology of the coatings. In contrast, the dissolution of alumina in strongly alkaline solutions [
62,
63] allow for an adjustment of the coating growth through the addition of hydroxide to the electrolyte [
4]. However, recent investigations indicate that this mechanism mainly affects the amorphous alumina phases, c.f. [
18].
4.3. Electric Regime
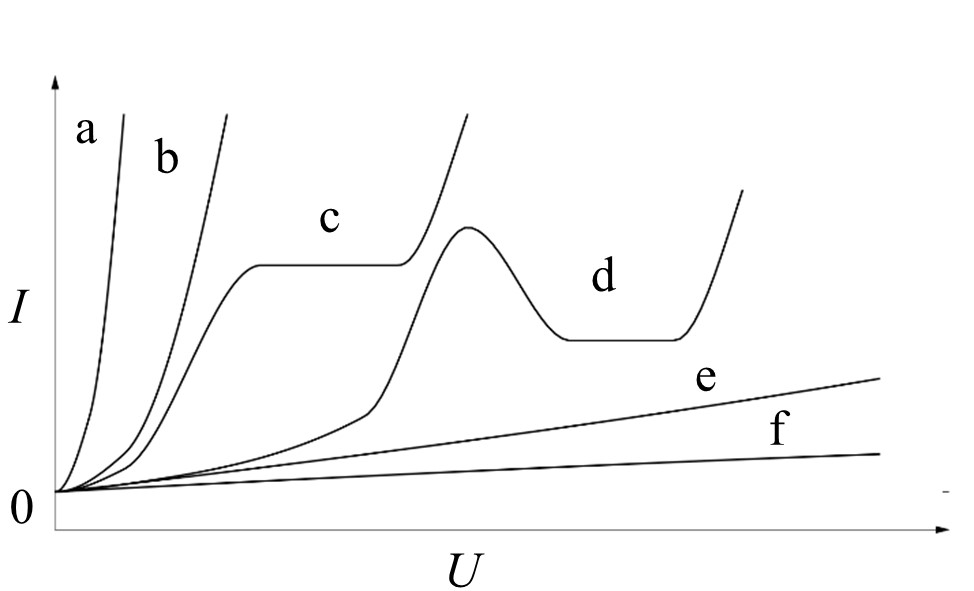
The electric regime during PEO can be determined by the control parameter (current density or cell voltage), the type of the supplied parameter (direct, alternating, pulse current/voltage), and the definition of the regime (frequency, breaks, limits etc.). Under direct current or voltage, the discharge events become ever more intense during the progression of the process. This includes large discharges with long life periods, which can have a deteriorating effect on both, the formed oxide and the substrate and thus lead to irreparable defects. This behavior results from excessive energy transfer and heat release. Therefore, pulse or alternating current or voltage regimes are used to limit the effect of the strong discharges and to facilitate the formation of thick oxide coatings up to a few hundred microns. Thus, the PEO process can be prolonged, the number of defects in the coatings decreased, and the formation of a thick technological layer on top of the coating is impeded. In the following, the effects of a pulsed regime and of an alternating regime shall be discussed.
In a pulse regime, the control parameter (current or voltage) is regularly set to a low value, typically to zero. During the formation of the barrier layer at the beginning of the process (the pre-spark stage), this only affects the process substantially, if the substrate material or the oxide are dissolved by the electrolyte.
The break in current flow regime gives time for chemical dissolution and can lead to a slower growth of the barrier oxide. In most cases, the barrier layer growth accelerates, since the break allows for the compensation of potential concentration gradients. Furthermore, the repeated steep rise of the control parameter results in a significant mass/charge transfer towards the substrate. After formation of the barrier layer, the system behaves like a capacitor. This includes that current flow occurs only above of a certain potential threshold. At each rising edge of the pulse, collision ionization is likely to occur and implies that the initiation of short, but intense discharge events are favored, as opposed to those favored in a direct current or voltage regime. At the falling edge of the pulse, the current flow is interrupted, and thus the length of the discharge periods are limited. Using this strategy, the PEO process can be performed for a longer duration without the development of large and deteriorating discharges. The utilization of an alternating regime likewise limits the life period of discharges. In addition, the cathodic half period of an alternating regime can lead to a partial electrochemical reduction of the oxide. Thus, the formation of a barrier layer sufficient for discharge initiation at the beginning of the PEO process will be prolonged. During the PEO process, discharge can also occur in the cathodic branch of the electric regime. This discharge is usually less intense than the discharge in the anodic branch. A lower amount of energy is introduced into the coating. However, the cathodic discharge is also prone to heat up the oxide locally, and are thus, likely to result in a reduction of the field strength necessary for the breakdown in the following anodic branch [
54].
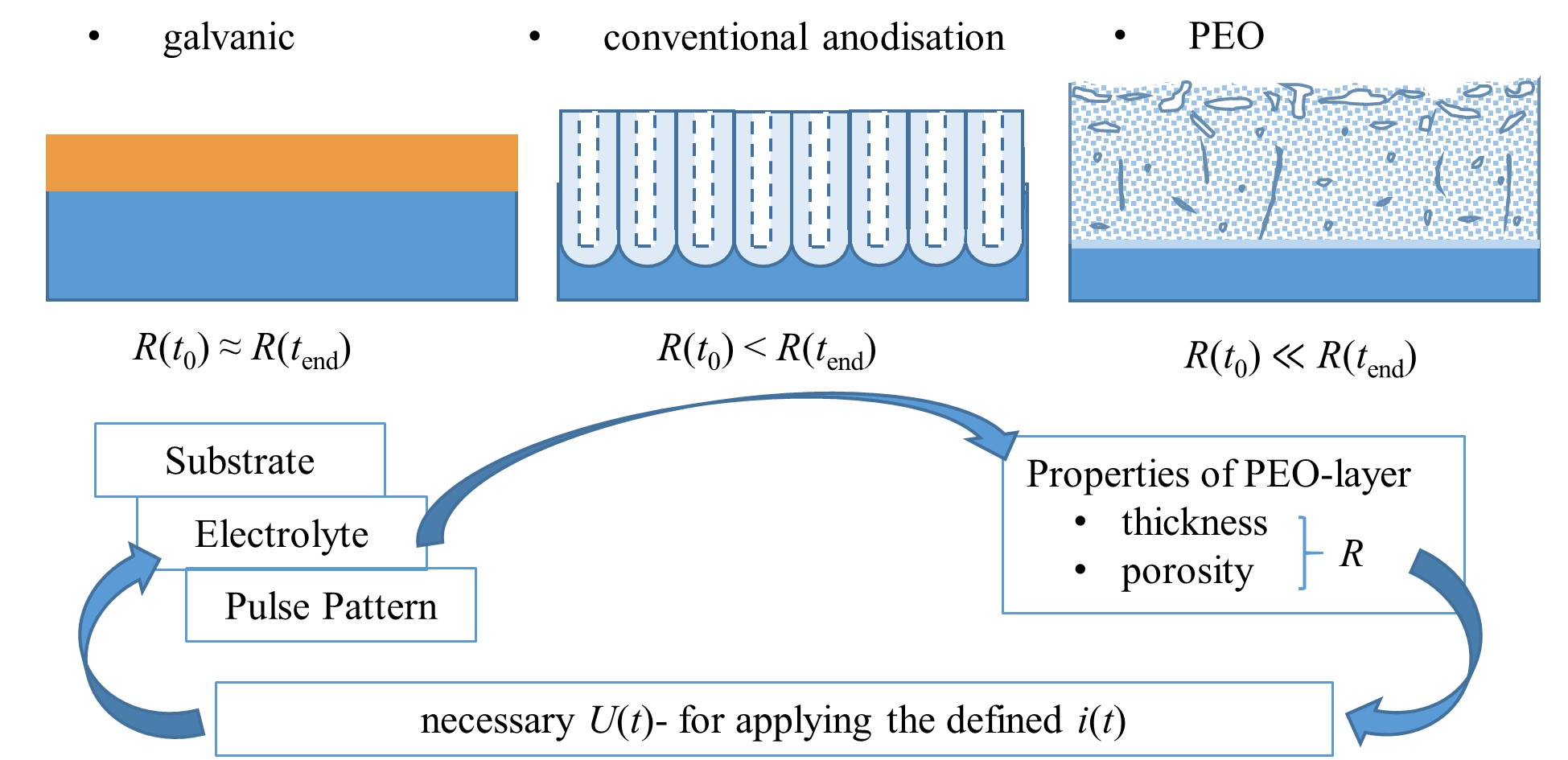
In general, the interactions of the substrate/electrolyte-combination with the electrical regime are complex and still a subject of research. A promising approach to gain experimental access to the relationships is the analysis of the electrical process data. Since, in contrast to other electrolytic surface treatment methods, PEO results in the formation of high-ohmic layers, these affect, above all in the case of current-controlled regimes, to what extent the pre-defined electrical pulse is mapped correctly in the experimental setup. shows a schematic representation of these relationships. illustrates them using the example of PEO of Mg-AZ31 samples with identical experimental parameters using different electrolytes.
Figure 7. Schematic representation (not to scale) of obtained-layer morphology and development of layer resistance R for different electrolytic coating methods relation to the process time t, interactions of the process variables voltage U(t) and current i(t) pattern with the layer characteristics during the PEO.
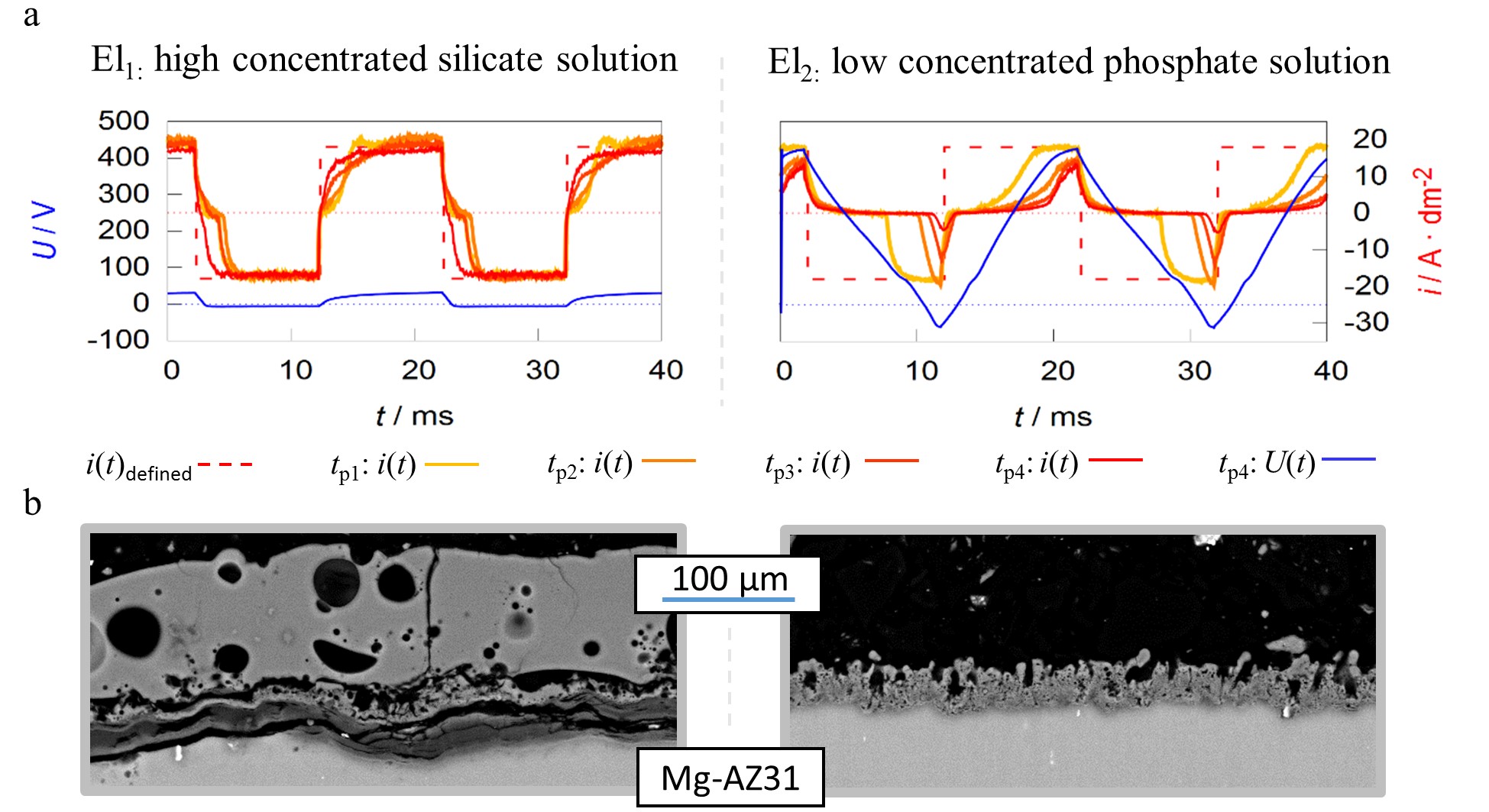
Figure 8. Pulse shape development of current-controlled PEO experiments for different process times (tp1–4 = 1, 5, 10 and 20 min) under identical experimental conditions in two different alkaline electrolytes El1/2 (a) SEM-micrographs of the resulting layers after 22:30 min of treatment time (b).
The working media used are designed for focused incorporation of electrolyte constituents into the coating (El1) and PEO layer formation by substrate conversion (El2). The current time characteristics measured during the process within the high concentration silicate solution show that the predefined symmetric rectangular current pattern is transmitted largely correctly to the system under investigation. Furthermore, the remaining deviations between predefined and measured pulses are decreasing with progression of the process time. In contrast to the effects described above, the experiment within the low concentration phosphate electrolyte shows a significant delay in the current flow after polarity reversal in both, the cathodic and anodic partial period. The delays even increase during the process. Additionally, the experiments in electrolyte 2 result in much higher voltages over the entire treatment time. This is exemplified by the voltage pattern after 20 min and leads to a higher consumption of electrical energy.
Therefore, one might assume that the experiment in electrolyte 1 had the more desirable process characteristics. However, consideration of the micrographs displayed in b show that, despite a significantly increased layer thickness, an insufficient layer adhesion was achieved within electrolyte 1. Conversely, the coating formed in electrolyte 2 shows a good substrate bonding. Thus, the more pronounced delay of the current flow with increasing process time is not an error in the control of the behavior of the rectifier but belongs to the PEO process characteristics. A good adhesive layer of ceramic leads to an increasing electrical resistance at the substrate/electrolyte interface, which indicates that the current flow only starts after the rectifier has readjusted to higher process voltages. If the layers produced adhere poorly or worse with increasing treatment time, this mechanism is suppressed.
This behavior is mentioned at this point in discussion because, in addition to the layer state, the ignition voltage for discharge initiation during the PEO is also influenced by the electrolyte composition. However, the electrolyte resistance (or electrolyte conductivity) is negligible in most cases [
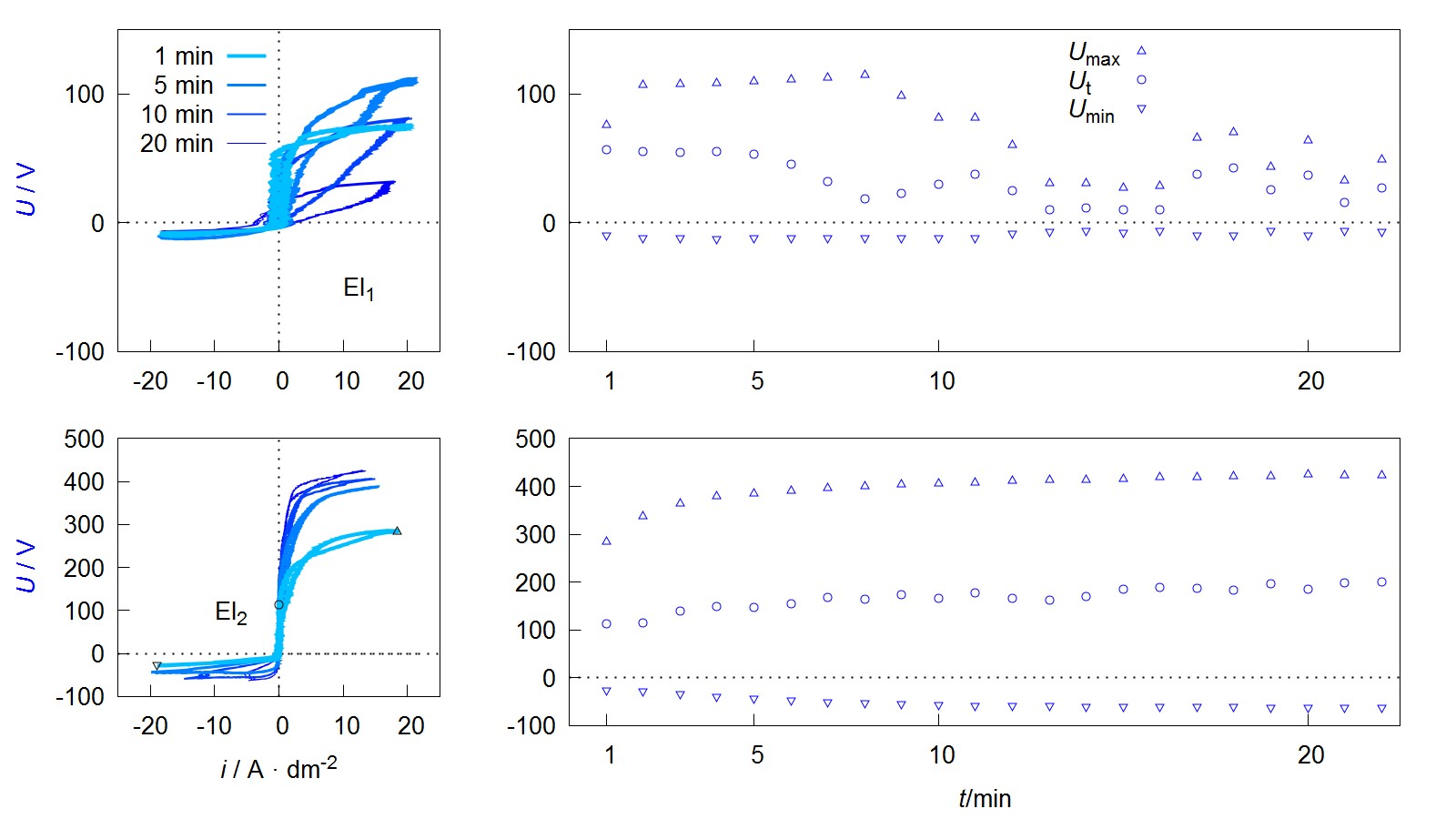
51]. The depiction of the electrical process variables in relation to each other allows further conclusions to be drawn about the underlying process characteristics. represents the process data shown in as voltage current curves (VCC), as well as the course of specific voltage values over the treatment time.
Figure 9. Voltage current curves of VCC (left) and course of maximum Umax, threshold Ut and minimum Umin voltage during the process (right) of the PEO experiments described in .
Depending on the form of the representation, these curves are also referred to as current-voltage curves or characteristics or more generally as oscillograms. The value of the maximum
Umax and minimum
Umin voltage during one pulse cycle can be easily extracted from such a diagram. Furthermore it is possible to see that the downward branch of the anodic period shows a characteristic point at which the current flow stops while the voltage is still above the coordinate origin. This behavior can be observed at the process data of PEO on aluminum under various conditions, shown in several publications [
64,
65,
66,
67,
68]. Within studies by Suminow et al. the value is called threshold voltage [
1,
2]. Duan et al. describes the polarization level below, when the charge carrier flow comes to a standstill as a critical conductive voltage [
69]. Both research groups investigate the course of this voltage in dependence of the treatment time and assume that the value is proportional to the electrical isolation properties of the PEO layer after collapse of the plasma electrolytic discharges. Therefore, an increasing
Ut indicates layer thickness growth and/or a decrease in the defect density within the layer. Insofar that this is correct, the evaluation of
Ut allows, with respect to the anti-corrosive properties of the resulting PEO layers, the determination of optimal treatment times. In addition, the workload for materialographic examinations are reduced, since
Ut provides automatically accessible information about the layer morphology. In contrast to microstructure images, these relate not only to a selected two-dimensional area but to the PEO layer along the entire substrate geometry. Hence, the method would be interesting also for non-destructive quality control.
Another interesting peculiarity of the VCCs shown in is that the ascending and descending anodic branches are not congruent. This is in accordance with recent studies by Ragov et al., which deal in detail with the analysis of electrical process data of the PEO of aluminum. Accordingly, the so-called hysteresis effects only occur in pulse regimes with cathodic components. The different conductivities in the rising and falling region of the anodic partial pulse are attributed to cathode-induced changes (CIC). These among others are substantiated by the incorporation of cations in a thin reaction layer at the substrate layer/interface of the so-called active zone. The subsequent release of the cations at the beginning of the anodic pulse leads to a brief increase in the electrical conductivity of the system. It is assumed that the few nm thick active zone has a strongly disproportionate contribution to the total electrical resistance of the layer system [
70,
71,
72].
This would limit the significance of the threshold voltage mentioned above. Provided that the results can be transferred to the PEO of magnesium, the more pronounced hysteresis effects in for electrolyte 1 can be explained by the fact that there are significantly more cations in the electrolyte and the cathodic partial pulses are mapped almost completely.