Microsurgical free flap reconstruction in acute burn care offers the option of reconstructing even challenging defects in a single stage procedure. Due to altered rheological and hemodynamic conditions in severely burned patients, it bears the risk of a higher complication rate compared to microsurgical reconstruction in other patients. To avoid failure, appropriate indications for free flap reconstruction should be reviewed thoroughly. Several aspects concerning timing of the procedure, individual flap choice, selection and preparation of the recipient vessels, and perioperative measures must be considered.
- burns
- microsurgery
- reconstruction
- free flap
1. Introduction
2. Indication
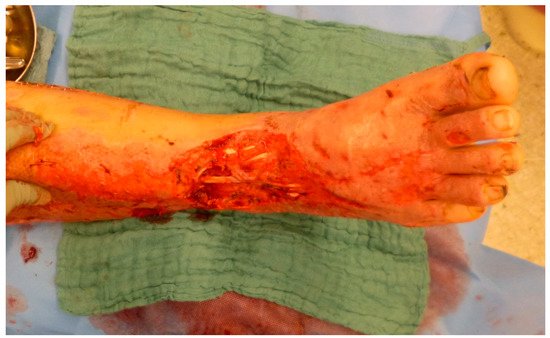
3. Timing
4. Flap Choice
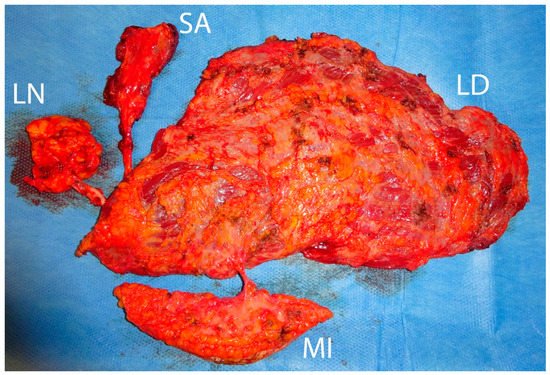
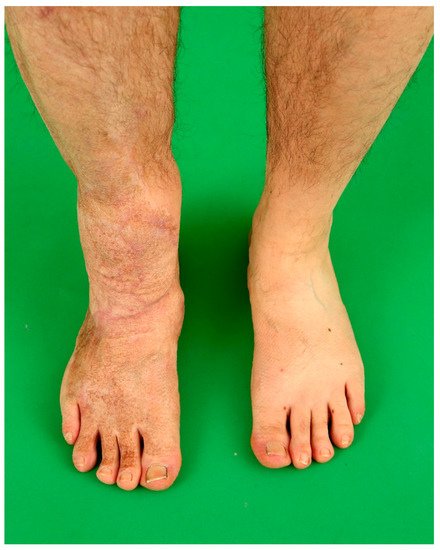
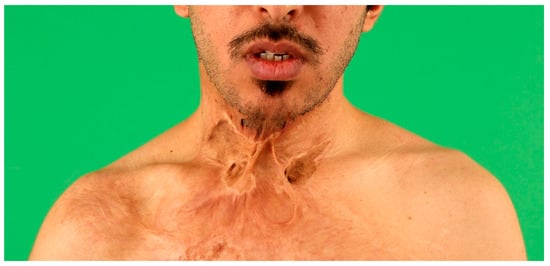
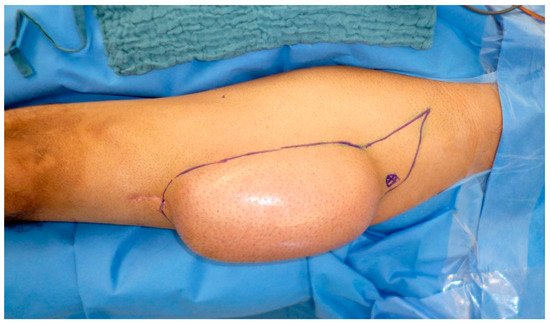
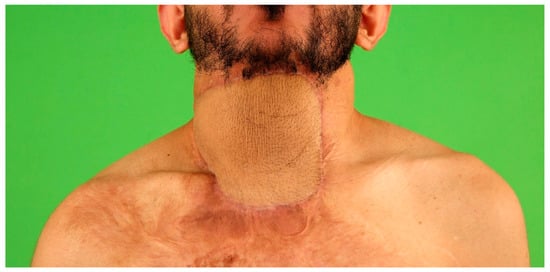
5. Recipient Site
6. Outcome and Complications
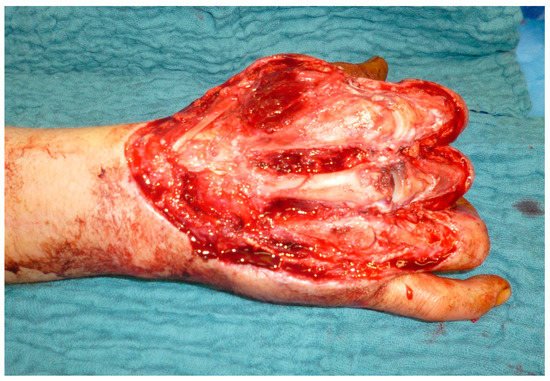
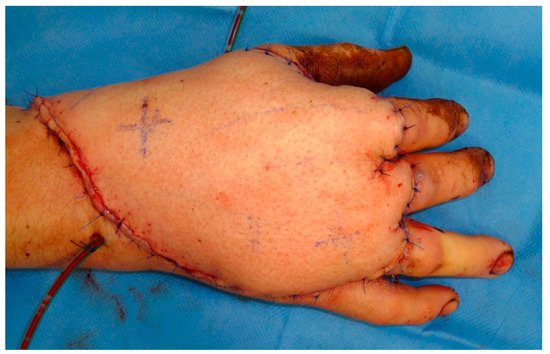
This entry is adapted from the peer-reviewed paper 10.3390/medicina57070718
References
- Cobbett, J.R. Free Digital Transfer. J. Bone Joint Surg. 1969, 51, 677–679.
- Van Zuijlen, P.P.; Van Trier, A.J.; Vloemans, J.F.; Groenevelt, F.; Kreis, R.W.; Middelkoop, E. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one-stage grafting model. Plast. Reconstr. Surg. 2000, 106, 615–623.
- Zelt, R.G.; Daniel, R.K.; Ballard, P.A.; Brissette, Y.; Heroux, P. High-voltage electrical injury: Chronic wound evolution. Plast. Reconstr. Surg. 1988, 82, 1027–1041.
- Klinkenberg, M.; Hellmich, S.; Germann, G.; Megerle, K.; Kolbenschlag, J. Impact of Timing of Admission and Microvascular Reconstruction on Free Flap Success Rates in Traumatic Upper Extremity Defects. J. Reconstr. Microsurg. 2015, 31, 414–419.
- Sauerbier, M.; Ofer, N.; Germann, G.; Baumeister, S. Microvascular reconstruction in burn and electrical burn injuries of the severely traumatized upper extremity. Plast. Reconstr. Surg. 2007, 119, 605–615.
- Schaden, E.; Hoerburger, D.; Hacker, S.; Kraincuk, P.; Baron, D.M.; Kozek-Langenecker, S. Fibrinogen function after severe burn injury. Burns 2012, 38, 77–82.
- Song, Y.G.; Chen, G.Z.; Song, Y.L. The free thigh flap: A new free flap concept based on the septocutaneous artery. Br. J. Plast. Surg. 1984, 37, 149–159.
- Hsiao, Y.-C.; Yang, J.-Y.; Chang, C.-J.; Lin, C.-H.; Chang, S.-Y.; Chuang, S.-S. Flow-through anterolateral thigh flap for reconstruction in electrical burns of the severely damaged upper extremity. Burns 2013, 39, 515–521.
- Schoenle, P.; Gazyakan, E.; Kremer, T.; Harhaus, L.; Kneser, U.; Hirche, C. The chimeric versatility of the subscapular system revisited: Backup options, coverage for bone transplants and vascularized lymph nodes. Plast. Reconstr. Surg. Glob. Open 2018, 6.
- Abramson, D.L.; Pribaz, J.J.; Orgill, D.P. The use of free tissue transfer in burn reconstruction. J. Burn Care Rehabil. 1996, 17, 402–408.
- Acartürk, T.O.; Bengür, F.B. Reconstruction of burn contractures of the anterior neck with pre-expanded free anterolateral thigh flaps. Injury 2020, 51.
- Hunt, J.L.; McManus, W.F.; Haney, W.P.; Pruitt, B.A. Vascular lesions in acute electric injuries. J. Trauma Inj. Infect. Crit. Care 1974, 14, 461–473.
- Henn, D.; Wähmann, M.S.; Horsch, M.; Hetjens, S.; Kremer, T.; Gazyakan, E.; Hirche, C.; Schmidt, V.J.; Germann, G.; Kneser, U. One-Stage versus Two-Stage Arteriovenous Loop Reconstructions: An Experience on 103 Cases from a Single Center. Plast. Reconstr. Surg. 2019, 143, 912–924.
- Platt, A.J.; McKiernan, M.V.; McLean, N.R. Free tissue transfer in the management of burns. Burns 1996, 22, 474–476.
- Aguilera-Saez, J.; Lopez-Masramon, B.; Collado, J.M.; Monte-Soldado, A.; Rivas-Nicolls, D.; Serracanta, J.; Barret, J.P. Severely damaged lower limb salvage in a critically ill burned patient. Lessons learned. Int. J. Burns Trauma 2020, 10, 191–200.
- Shen, T.Y.; Sun, Y.H.; Cao, D.X.; Wang, N.Z. The use of free flaps in burn patients: Experiences with 70 flaps in 65 patients. Plast. Reconstr. Surg. 1988, 81, 352–357.
- Uslu, A. Reconstruction of the Distal Leg and Foot Using Free Anterolateral Thigh Flaps in Patients with High-Voltage Electrical Burns. J. Burn Care Res. 2019, 40, 703–709.
