Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Coatings & Films
Thin polymer films play critical roles in various glass industry applications. They have been used as adhesives in automotive and architecture, an anti-fouling coating layer in touch-screen applications, a substrate for organic light emitting diode, and a protective layer for glass packaging.
- polymer film
- adhesion
- bonding mechanism
- surface morphology
- relative humidity
1. Introduction
Required interfacial properties are widely varied depending on the purpose of the coating on the surface. For example, anti-fouling layer requires the hydrophobic/oleophobic property at the interface with high adhesion strength for durability on glass surface [1]. On the other hand, polyimide thin film as a carrier for display glass needs a moderate level of adhesion with the glass surface, which can prevent failure at the interface, as well as allow easy detachment if needed [2][3]. In the case of a glass-surface protection layer, adhesion of polymer layer on the glass is sufficient so long as it can prevent stiction of dust particles or stains. However, the layer should be completely removable by a simple process, such as washing, for easy handling after transportation. Consequently, understanding the interfacial behavior is of primary importance for the development of polymer coating for target goals.
Generally, polymer films are easily deformed, and thereby can form many kinds of interfaces with physical and chemical interactions [4][5][6][7]. For instance, polymeric chains can be diffused into the porous and irregular surface of the substrate and entangle with each other, leading to a mechanically interlocked interface. In cases of epoxy resin or rubber materials, additional heating may induce crosslinking between entangled chains to form harder locking. If surface species of the counterpart substrate have high mobility, an interdiffusion layer can also be formed. However, typical interfacial interactions explained above are significantly limited by surface properties of the glass substrate. It is because polymer chains are hard to diffuse into the glass surface without additional engineering due to low surface roughness and porosity, as well as high cohesive strength of glass [8]. In addition, the strong tetrahedral network of silicate glass maintains its hardness up to a glass transition temperature where the polymer is completely degraded, which implies formation of an interdiffusion layer between glass and polymer at room temperature is almost impossible. Therefore, the most typical way of interface processing would be to utilize intermolecular interactions between the polymer and glass elements on the surface [9]. However, it is notable that there can be various kinds of interactions for polymer–glass interfaces following types of intermolecular force encompassing from weak ‘physical’ interactions to strong ‘chemical’ bonding [10].
Atomistic modeling techniques have proved to be powerful tools for studying the mechanisms of interfacial behavior in molecular scale [11][12][13][14][15][16][17]. Density functional theory (DFT) provides electronic structure of molecules, which gives us an intrinsic information for chemical affinity at the interface. In addition, molecular dynamics (MD) simulation describes a model on the length scale from nanometer to micrometer, and thus both conformational changes of polymers and adhesion/detach process of a film can be analyzed. Chemical bonding and physical interactions mentioned above are often referred to as ‘bonded’ and ‘non-bonded’ interactions in the sense that physical interactions do not ‘tie’ two atoms specifically, rather gather all atoms of the group loosely [10][18]. Non-bonded interactions usually include van der Waals forces, polar interactions including hydrogen bonds, and Coulomb interaction.
Often, interfacial adhesion and relevant failure behavior are not easy to understand because the above interactions contribute to the adhesion together. MD simulation can decompose energy terms of non-bonded interactions to track their separate roles for adhesion strength. In this way, it is able to consider possible factors which affect the adhesion such as effects of rigidity of the polymer film and ratio of polar functional groups. Effects of adhesion measurement methods can be also analyzed through simulated adhesion tests with various modes [19][20][21][22]. Meanwhile, the bonded interaction includes strong covalent and ionic bonding. For example, one can form covalent bonding between silane end groups of the specially prepared polymer and silanol groups on the glass surface to greatly improve interfacial adhesion. DFT calculations for such interfaces can reproduce precise steps of bonding formation under hydrolysis and condensation process, and the resultant bonding strength can be also calculated [23]. MD simulation clarifies nanoscale characteristics of the interface, such as the adhesion strength, thickness, and surface density of the polymer film [24]. Furthermore, for both cases of bonded and non-bonded interaction, atomistic modeling can artificially control the extrinsic conditions, such as the surface morphology of the glass substrate and relative humidity, to study their unique effects on the adhesion [23][24][25][26][27].
2. Extrinsic Conditions Affecting Interactions
2.1. Surface Morphology: Effect of Nanoscale Roughness on the Adhesion
Nanoscale roughness exhibits critical role for the adhesion of interfaces with both non-bonded and bonded interactions. For the non-bonded interaction, hydroxylation density on silica surface was one of the key parameters for adhesion of PI [19], and thus it is a practical way to enhance adhesion by increasing hydroxyl density with surface roughness. For the bonded interaction, surface reaction at the interface determines molecular density and hence, roughness is a parameter of interest that impacts adhesion. As described in Section 2.3, ordered roughness is defined by amplitude and spacing (Figure 2) [28], and the effects of individual parameters on the adhesion are investigated for both PI and SPFPE adsorption on the silica surface [24][25].
Figure 12a shows adhesion energy variation for PI–glass interface depending on surface roughness, and roughness amplitude (Ra) exhibits the highest impact on the adhesion. Roughness spacing corresponds to the period of ordered roughness, and it is important to mention that the impact of amplitude is also determined by the roughness period [25]. As the roughness period decreases, un-contacted area between PI and glass increases with formation of vacant pores, and hence effect of amplitude on the adhesion is reduced. As roughness period increases, the two surfaces are well-attached to each other, and adhesion energy is maximized when the period is the longest (see Figure 12a). However, a further increase of spacing implies reduction of the surface area, and thus there exists an optimal roughness spacing for maximal adhesion strength. In addition, the energetic contribution to the adhesion is analyzed by energy decomposition during the pulling process, and it is revealed that not only is there an increase of hydrogen bond energy but also the increase of Coulomb energy contributes to the adhesion energy variation by roughness. The contribution of Coulomb energy becomes more significant when roughness is higher [25].
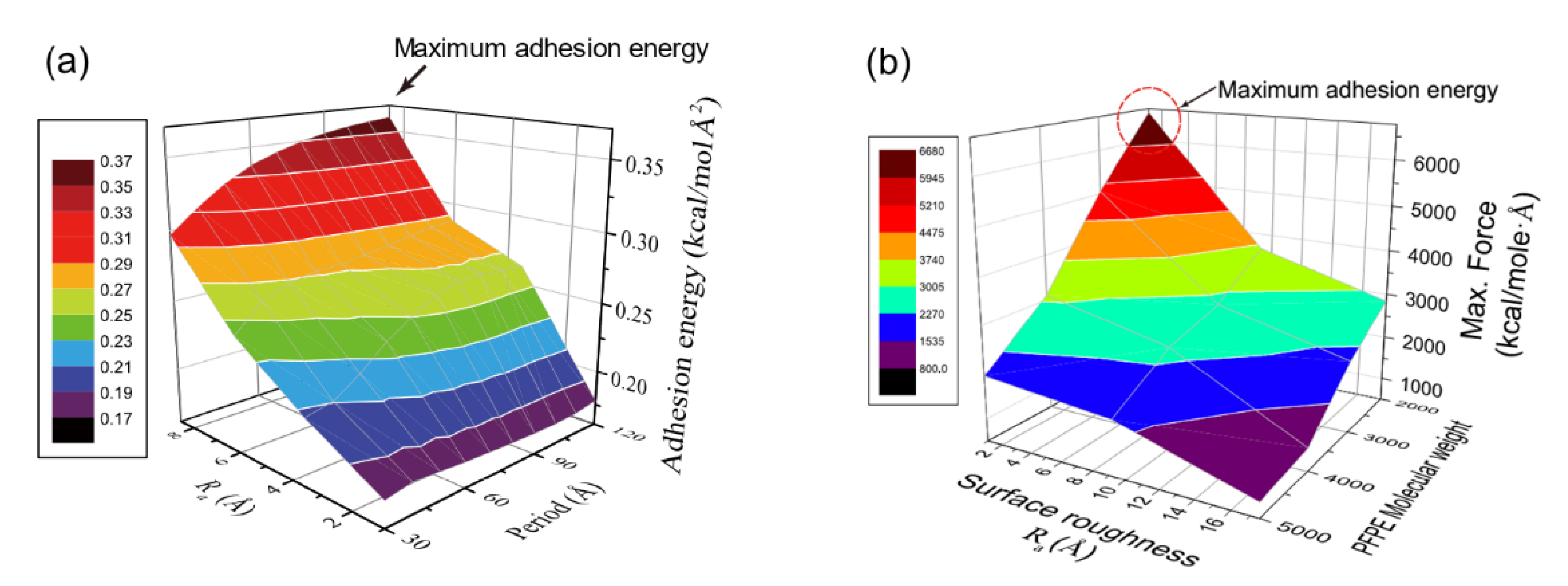 Figure 12. Variation of adhesion energy for (a) PI–glass and (b) SPFPE–glass interface (adapted with permission from References [24][25], Copyright 2019 and 2017 American Chemical Society).
Figure 12. Variation of adhesion energy for (a) PI–glass and (b) SPFPE–glass interface (adapted with permission from References [24][25], Copyright 2019 and 2017 American Chemical Society).2.2. Humidity
Humidity is one of the dominant environmental factors which hugely affects interfacial properties because of its ability to modify the surface structure of glass, as well as the interface between coating material and glass. It is known that only a few water molecules can induce hydroxylation of the glass surface, and water amounts corresponding to 15% relative humidity are enough to form a hydrated surface with a monolayer of water [29]. Furthermore, water molecules can diffuse into the polymer film and modify flexibility of the chain, thereby affecting adhesion too; therefore, the effect of humidity on the interfacial properties is of great importance.
3. Conclusions
Interfacial interactions and adhesion behavior of polymer–glass interfaces were discussed, with a focus on atomistic simulation methods. Various types of polymers, such as homopolymers, copolymers, natural polymers, and surfactants, were considered, and depending on the surface adsorption behavior, polymer-glass interactions were classified as non-bonded and bonded interactions. In the works for non-bonded interaction, three main interactions, namely van der Waals, polar, and hydrogen bonds, were reviewed, and the contributions to interfacial adhesion energy were extensively analyzed. It was revealed that the dominant interaction for adhesion is hydrogen bonding due to hydroxyl groups from both the polymer molecules and the glass surface. In addition, it was found that the flexibility of the polymer chain and modes of adhesion test can affect adhesion and failure behavior at the interface. In the case of bonded interactions, creation of covalent siloxane bonds between silane groups in the polymer and hydroxyl groups on the glass surface are critical for strong interfacial interaction. A detailed mechanism of covalent bond formation was described, and adhesion properties, along with molecular density analysis, were reviewed with an example of SPFPE.
One finds that parallel orientation of SPFPE is observed and only a single siloxane bond is formed among three silanol groups in the branch of SPFPE. Therefore, molecular density and thickness of the film are very low compared to the conventional self-assembled monolayer molecules. It is suggested that one effective way to enhance adhesion is to increase the molecular density of SPFPE rather than increasing the number hydroxyl groups on the silica. Besides interfacial interactions, external conditions, such as the surface morphology of the glass substrate and relative humidity, yield significant effects on the interfacial adhesion. For example, modulation of amplitude of surface roughness is most critical to the adhesion regardless of bonding type. In addition, the introduction of water molecules at the interface not only forms additional amounts of hydrogen bonds but also makes the polymer film more rigid, and thus the level of adhesion can be drastically different compared to the interface in the absence of water. In summary, comprehensive insights into the interfacial bonding mechanism of adhesion and failure behavior obtained from computational studies can be used for surface engineering purposes. It is also possible to extend such methodologies and concepts to other kinds of polymer-glass interfacial systems, as well as to understand adhesion of organic-inorganic interface in general.
This entry is adapted from the peer-reviewed paper 10.3390/polym13142244
References
- Moore, E.; Delalat, B.; Vasani, R.; McPhee, G.; Thissen, H.; Voelcker, N.H. Surface-Initiated Hyperbranched Polyglycerol as an Ultralow-Fouling Coating on Glass, Silicon, and Porous Silicon Substrates. Appl. Mater. Interfaces 2014, 6, 15243–15252.
- Chaudhury, M.K.; Gentle, T.M.; Plueddemann, E.P. Adhesion Mechanism of Polyvinyl Chloride to Silane Primed Metal Surfaces. J. Adhesion Sci. Tech. 1987, 1, 29–38.
- Juhl, K.M.; Bovet, N.; Hassenkam, T.; Dideriksen, K.; Pedersen, C.S.; Jensen, C.M.; Okhrimenko, D.V.; Stipp, S.L. Change in Organic Molecule Adhesion on α Alumina (Sapphire) with Change in NaCl and CaCl2 Solution Salinity. Langmuir 2014, 30, 8741–8750.
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of Polymers. Prog. Polym. Sci. 2009, 34, 948–968.
- Bhushan, B. Adhesion and Stiction: Mechanisms, Measurement Techniques, and Methods for Reduction. J. Vac. Sci. Technol. B 2003, 21, 2262–2296.
- Lacomb, R. Adhesion Measurement Methods Theory and Practice; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; Volume 1, pp. 7–74.
- Hudzinskyy, D.; Lyulin, A.V.; Baljon, A.R.C.; Balabaev, N.K.; Mechels, M.A.J. Effects of Strong Confinement on the Glass-transition Temperature in Simulated Atactic Polystyrene Filmes. Macromolecules 2011, 44, 2299–2310.
- Varshneya, A.K. Fundamentals of Inorganic Glasses, 2nd ed.; Society of Glass Technology: Sheffield, UK, 2013; Volume 1, pp. 477–524.
- Miwa, T.; Tawata, R.; Numata, S. Relationship between Structure and Adhesion Properties of Aromatic Polyimides. Polymer 1993, 34, 621–624.
- Israelachvili, J.N. Intermolecular and Surface Forces; Elsevier: Waltham, MA, USA, 2011; Volume 1, pp. 23–52.
- Marotzke, A.; Hampe, C. The Energy Release Rate of the Fiber/Polymer Matrix Interface: Measurement. J. Reinf. Plast. Compos. 1997, 16, 341–352.
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology, Revised and Expanded; CRC Press: New York, NY, USA, 2003; pp. 159–180.
- Pham, T.A.; Lee, D.; Schwegler, E.; Galli, G. Interfacial Effects on the Band Edges of Functionalized Si Surfaces in Liquid Water. J. Am. Chem. Soc. 2014, 136, 17071–17077.
- Wippermann, S.; Voros, M.; Gali, A.; Gygi, F.; Zimanyi, G.T.; Galli, G. Solar Nanocomposites with Complementary Charge Extraction Pathways. Phys. Rev. Rett. 2014, 112, 106801.
- Muhich, C.L.; Qui, J.; Holder, A.M.; Wu, Y.C.; Weimer, A.W.; Wei, W.D.; McElwee-White, L.; Musgrave, C.B. Solvent Control of Surface Plasmon-Mediated Chemical Deposition of Au Nanoparticles from Alkylgold Phosphine Complexes. ACS Appl. Mater. Interfaces 2015, 7, 13384–13394.
- Gleizer, A.; Peralta, G.; Kermode, J.R.; De Vita, A.; Shermann, D. Dissociative Chemisorption of O2 Inducing Stress Corrosion Cracking in Silicon Crystals. Phys. Rev. Rett. 2014, 112, 115501.
- Ren, J.; Zhou, G.F.; Guo, Z.C.; Zhang, W. Density Functional Theory Study on the Surface Reaction Mechanism of Atomic Layer Deposited Ta2O5 on Si(100) Surfaces. Chem. J. Chin. Univ. 2009, 30, 2279–2283.
- Heinz, H.; Lin, T.-J.; Mishra, R.K.; Emami, F.S. Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field. Langmuir 2013, 29, 1754–1765.
- Goyal, S.; Park, H.-H.; Lee, S.H.; Savoy, E.; McKenzie, M.E.; Rammohan, A.R.; Mauro, J.C.; Kim, H.; Min, K.; Cho, E. Characterizing the Fundamental Adhesion of Polyimide Monomers on Crystalline and Glassy Silica Surfaces: A Molecular Dynamics Study. J. Phys. Chem. C 2016, 120, 23631–23639.
- Min, K.; Kim, Y.; Goyal, S.; Lee, S.H.; McKenzie, M.E.; Park, H.; Savoy, E.; Rammohan, A.R.; Mauro, J.C.; Kim, H. Interfacial Adhesion Behavior of Polyimides on Silica Glass: A Molecular Dynamics Study. Polymer 2016, 98, 1–10.
- Min, K.; Rammohan, A.R.; Lee, H.S.; Shin, J.; Lee, S.H.; Goyal, S.; Park, H.; Mauro, J.C.; Stewart, R.; Botu, V.; et al. Computational Approaches for Investigating Interfacial Adhesion Phenomena of Polyimide on Silica Glass. Sci. Rep. 2017, 7, 10475.
- Min, K.; Rammohan, A.R.; Lee, S.H.; Goyal, S.; Park, H.; Stewart, R.; He, X.; Cho, E. Grafting Functional Groups in Polymeric Binder toward Enhancing Structural Integrity of LixSiO2 Anode during Electrochemical Cycling. J. Phys. Chem. C 2018, 122, 17190–17198.
- Ahn, Y.N.; Lee, S.H.; Oh, S.Y. Adsorption characteristics of silane-functionalized perfluoropolyether on hydroxylated glassy silica surfaces: A multiscale approach. Appl. Surf. Sci. 2019, 496, 143699.
- Lee, S.H.; Ahn, Y.N.; Botu, V.; Stewart, R.J.; Oh, S.Y. Enhancement of Adhesion Strength of Perfluoroalkylpolyethers on Rough Glassy Silica for Antismudge Coatings. ACS Appl. Polym. Mater. 2019, 1, 2613–2621.
- Lee, S.H.; Stewart, R.J.; Park, H.; Goyal, S.; Botu, V.; Kim, H.; Min, K.; Cho, E.; Rammohan, A.R.; Mauro, J.C. Effect of Nanoscale Roughness on Adhesion between Glassy Silica and Polyimides: A Molecular Dynamics Study. J. Phys. Chem. C 2017, 121, 24648–24656.
- Park, H.; Lee, S.H. Roles of Paper Composition and Humidity on the Adhesion between Paper Sheet and Glass: A Molecular Dynamics Study. Cellulose, Under review.
- Park, H.; Lee, S.H.; Acquard, D.F.; Agnello, G.; Banerjee, J. Computational Analysis on the Adhesion Mechanism between Organic Coatings and Glass by Molecular Dynamics Simulations. In Proceedings of the International Congress on Glass, Boston, MA, USA, 9–14 June 2019.
- Bhushan, B. Modern Tribology Handbook; CRC Press: Boca Raton, FL, USA, 2001; Volume 1, pp. 40–52.
- Verdaguer, A.; Weis, C.; Oncins, G.; Ketteler, G.; Bluhm, H.; Salmeron, M. Growth and Structure of Water on SiO2 Films on Si Investigated by Kelvin Probe Microscopy and in Situ X-Ray Spectroscopies. Langmuir 2007, 23, 9699–9703.
This entry is offline, you can click here to edit this entry!
