Fungal co-infections are reported in severely ill COVID-19 patients admitted to the ICU, with a higher rate of incidence for aspergillosis followed by candidemia, as observed from our literature analysis. Fungal co-infections may increase disease severity and lead to more severe outcomes.
- COVID-19
- fungal co-infections
- corticosteroid treatment
- COVID-19-associated candidiasis
- COVID-19-associated pulmonary aspergillosis
- mucormycosis
Incidence and prevalence
Hospitalized COVID-19 patients in the ICU may encounter other complications such as secondary microbial infections due to pathogenic molds and yeasts. In multiple centers across Wales, an incidence of 14.1% and 12.6% for aspergillosis and yeast infections, respectively, were observed amongst critically ill COVID-19 patients.[1] An analysis of clinical data from several countries revealed that the aggregate incidence of COVID-19-associated pulmonary aspergillosis (CAPA) was found to be between 1% and 39.1%[2]. Whereas, for candidemia (blood infections of Candida spp.), up to 12% were reported for a health center[3] and between 1.54% and 7.54% for a center in Brazil [4]. In order to elucidate the diversity and co-occurrence of fungal co-infections in COVID-19 patients, we summarized several randomly selected reports on fungal co-infections in COVID-19 patients (Figure 1).
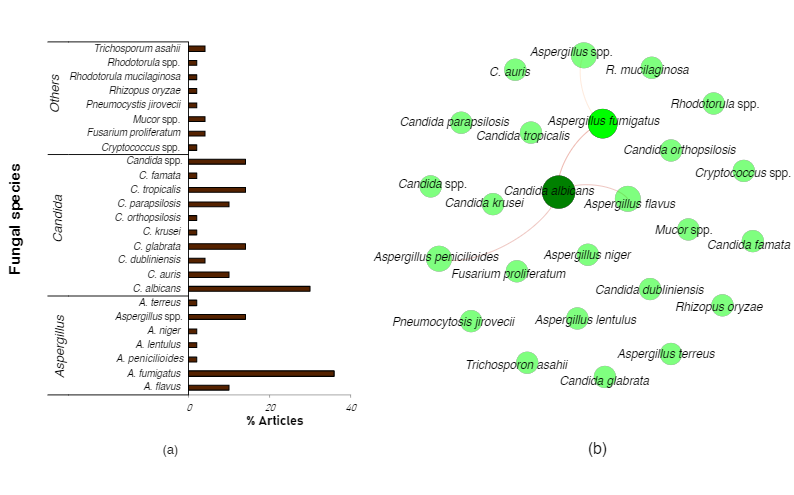 Our non-exhaustive summary of the literature reports revealed at least twenty different fungal species in hospitalized COVID-19 patients. Most of the fungal co-infections are due to Aspergillus fumigatus (common etiological agent of COVID-19 associated pulmonary aspergillosis (CAPA)), followed by Candida albicans (common etiological agent of candidiasis or candidemia) (Figure 1a). Significant co-occurrence between Candida albicans and Aspergillus fumigatus, A.flavus, or A.penicillioides was mostly reported (Figure 1b).
Our non-exhaustive summary of the literature reports revealed at least twenty different fungal species in hospitalized COVID-19 patients. Most of the fungal co-infections are due to Aspergillus fumigatus (common etiological agent of COVID-19 associated pulmonary aspergillosis (CAPA)), followed by Candida albicans (common etiological agent of candidiasis or candidemia) (Figure 1a). Significant co-occurrence between Candida albicans and Aspergillus fumigatus, A.flavus, or A.penicillioides was mostly reported (Figure 1b).
Contribution to Disease Severity, Multidrug-Resistant fungi, and Mucormycosis
Prospective and retrospective data of COVID-19 patients admitted to intensive care units (ICU), especially for a prolonged duration, show that these patients are susceptible to invasive microbial co-infections during hospitalization and that these may lead to more severe outcomes [1][5][6][7]. A prospective cohort study of 135 adults, performed across multiple centers in Wales, showed a significantly higher (up to 25%) mortality rate in COVID-19 patients with fungal infections compared to patients without fungal infections [1]. In particular, a multicenter study of 108 COVID-19 patients admitted to the ICUs in Italy showed a significantly higher 30-day mortality rate for patients with probable COVID-19-associated pulmonary aspergillosis (CAPA) or putative invasive pulmonary aspergillosis, compared to patients without suspected aspergillosis [8]. Similarly, Meijer and co-workers [9] reported mortality between 40% and 50% in patients with CAPA across the first wave (March–April 2020) and second wave (mid-September to mid-December 2020) of the COVID-19 pandemic in Brazil.
With respect to COVID-19-associated candidiasis (CAC), although the incidence rates may be slightly lower than that of CAPA, the mortality rate of COVID-19 patients with candidemia does not appear to differ markedly. Reports from Italy indicate that up to 57.1% and 50% mortality was reported in COVID-19 patients with candidemia [10] and Candida auris candidemia [11], respectively. Elsewhere, the mortality rate of COVID-19 patients with candidemia exceeded those of counterparts without candidemia in Iran (100% vs. 22.7%)[7]. Altogether, these observations highlight and re-emphasize the propensity of fungal co-infections to exacerbate disease severity and, consequently, increase the mortality of critically ill patients admitted to the ICUs.
Co-infections by antifungal resistant fungi have also been reported amongst hospitalized COVID-19 patients. Indeed, microbial-resistant pathogens might be consequential for COVID-19 prognosis. In a study[12], the hazard ratio for death within 90 days in critically ill COVID-19 patients was significantly increased by antimicrobial-resistant pathogens. A report[13] from India stated a case-fatality rate of 60% amongst COVID-19 patients with candidemia due to multidrug-resistant C. auris infection. Hence, given that antifungal resistance undermines treatment efforts and can escalate treatment costs, reports of fungal species with resistance to multiple antifungals[14][13][11], including echinocandins[15], are worrying. Furthermore, these reports highlight the need to appreciate the global burden of fungal co-infections in the current COVID-19 pandemic and the importance of prompt diagnosis and treatment of fungal pathogens in hospitalized COVID-19 patients.
Furthermore, management of COVID-19 and other underlying host factors have exacerbated the incidence of mucormycosis. Mucormycosis has been reported mostly among COVID-19 survivors (although cases in currently hospitalized COVID-19 patients have also been observed)[16]. Rhizopus arrhizus appears to the most common etiological agent of COVID-19-associated mucormycosis (CAM) in India [17]. However, Rhizopus microsporus, Rhizopus homothallicus, Mucor irregulars, Saksenaea erythrospora, and Apophysomyces variabilis have also been implicated in mucormycosis cases in India and elsewhere [17][18][19]. A recent systematic review of mucormycosis cases in India and worldwide reported that corticosteroid use was recorded in 76.3% of cases and that 30.7% of mucormycosis cases were fatal [20]. Altogether, the foregoing reports and several other studies suggest that the heightened incidence of mucormycosis in India is related to certain risk factors, including poorly managed diabetes and the prolonged usage of high dosage steroids in treating COVID-19 [21][22][23].
Overview of Risk Factors for Opportunistic Fungal Infections in Critically Ill COVID-19 Patients
Overall, risk factors driving the high incidence of aspergillosis and candidemia in COVID-19 patients are related to invasive procedures (e.g., intubation) predisposing lung tissues to fungal colonization and proliferation [24][25][26], history of chronic pulmonary disease [1], prolonged corticosteroid treatments [1][26], immunological disposition of patients and antimicrobial therapy [15][27]. In one study comparing co-infections in critically ill patients with and without COVID-19, it was observed that the need for invasive assisted respiration was the most decisive factor for co-infections with antifungal-resistant pathogens in patients with severe COVID-19 [24].
Given the high incidence of CAPA and the distinct clinical features of CAPA compared to influenza-associated pulmonary aspergillosis, it has been necessary to establish appropriate case definitions for CAPA in order to facilitate uniformity of reporting across medical practices. To this end, a number of case definitions or guidelines have been proposed for characterizing possible, putative, probable, and proven CAPA cases (See [1][28][29]). According to Koehler and co-workers [28], a proven case of CAPA may be established by direct microscopic and/or histopathological evidence of fungal features that are typical of Aspergillus spp. Such evidence includes an observation of invasive growth into tissues with concomitant tissue damage, recovery of Aspergillus spp.
SARS-CoV-2 insults in the lungs elicit the release of danger-associated molecular patterns (DAMPs) in severe COVID-19 disease [25]. The release of DAMPs is accompanied by inflammation and extensive damage of lung epithelial tissues, which are enabling risk factors for invasive pulmonary aspergillosis [25]. In severe COVID-19 disease, extensive inflammation and injury to the lungs lead to acute respiratory syndrome (ARDS). ARDS is characterized by difficulty in breathing; hence, assisted ventilation is required for such patients. However, mechanical ventilation and the duration of ventilation is a known risk factor for invasive aspergillosis and CAPA in the ICU [30][31][32].
In addition, pharmaceutical treatments for malignancy and the use of corticosteroids (discussed in a later section) and antibiotics may be risk factors for CAPA [1][33]. For example, in a multicenter study across Wales [1], a significant association was observed between COVID-19 patients with IPA and patients treated for or diagnosed with solid malignancy. Also, treatment with azithromycin for up to 3 days significantly correlated with the incidence of probable invasive pulmonary aspergillosis in COVID-19 patients [33]. Such observation was attributed to the immunomodulatory properties of azithromycin that may weaken the host’s immune response and subsequent susceptibility to aspergillosis [34].
COVID-19-associated candidiasis (CAC) refers to the detection of one or more
Candida spp. in the bloodstream or body tissues of COVID-19 patients. Some of the risk factors identified for CAC include prolonged hospital stays, mechanical ventilation, central venous catheters, surgical procedure, and the use of broad-spectrum antibiotics [4][35]. It was observed that COVID-19 patients with candidemia were more likely to be under mechanical ventilation than non-COVID-19 patients [35]. Also, COVID-19 patients with candidemia were more likely to be in the ICU and receiving immunosuppressive agents than patients in the ICU for reasons other than COVID-19 [10].
Immunosuppressants and Fungal co-infections in Critically Ill COVID-19 Patients
Most of the current treatment options for managing patients with severe COVID-19 are immunomodulators [36]. The anti-inflammatory properties of these immunomodulators are important to counteract the heightened and unregulated release of pro-inflammatory cytokines (also known as ‘cytokine storm’) in the lungs during SARS-CoV-2 infection [37][38]. Thus, immunosuppressants such as dexamethasone, methylprednisolone, prednisone, hydrocortisone, and tocilizumab constitute the most common treatment options for managing severe COVID-19 cases in the ICU [36]. For example, dexamethasone treatment decreased the 28-day mortality in COVID-19 patients on invasive respiratory support or receiving oxygen alone but was not particularly beneficial for COVID-19 patients with less severe disease, suggesting that hyper inflammation mediates the advanced stage of the disease and therefore can be alleviated by immunosuppressants [39][40].
Unfortunately, the immunosuppressants hamper both the individual’s innate and adaptive immune responses through sophisticated quantitative and qualitative mechanisms of immune deregulation [33][41][42][43][44], thereby increasing patients’ susceptibility to invasive fungal diseases. In particular, steroidal immunosuppressants such as corticosteroids predominantly affect the protective immunity process qualitatively through functional impairment of several effector immune cells, such as monocytes, polymorphonuclear leukocytes, T lymphocytes, and macrophages [42] and is a significant acquired immunological risk factor for pulmonary aspergillosis [45][46]. Thus, corticosteroids such as dexamethasone and methylprednisolone, used for managing critically ill COVID-19 patients, have contraindications, including fostering secondary microbial infections in patients [45][47].
In the present COVID-19 pandemic, questions are being asked regarding the possible relationship between immunosuppressant or corticosteroid use and the incidence of fungal infections in critically ill COVID-19 patients. A number of studies investigating the link between immunosuppressants and fungal co-infections in hospitalized COVID-19 patients are summarised in our review paper (see [48]). In one study [49], a 10-fold increase in candidemia amongst a cohort of critically ill COVID-19 patients receiving high doses of corticosteroids such as prednisone, hydrocortisone, methylprednisolone, and dexamethasone was observed. Similarly, a retrospective study conducted in Chicago and involving 111 COVID-19 patients receiving tocilizumab (a monoclonal antibody that inhibits binding of IL-6 to the membrane and soluble receptors [50]) was significantly linked with the risk of developing fungal pneumonia and sinusitis [51]. However, in a retrospective study involving 4313 COVID-19 patients in New York, corticosteroid use was not associated with increased bacteremia or fungemia compared to non-corticosteroid users when administered within the first seven days of admission [52]. It must, however, be noted that in many of the other reports, mention is made of high doses of corticosteroids often administered for prolonged periods [53], which may explain the high incidence of systemic fungal infections and ultimately negate the lifesaving benefits of these drugs.
It should also be noted that the correlation between corticosteroid use and incidence of fungal infections in hospitalized COVID-19 patients may be masked by other co-founding risk factors for fungal infections, such as the patient’s history of pulmonary disease, comorbidities, and mechanical ventilation [1][54]. Apart from corticosteroid use, a history of chronic respiratory disease was linked to a significant increase in the likelihood of aspergillosis [1]. Unfortunately, most of the currently reported investigations on the potential role between immunosuppressants and fungal infections are from a small cohort of patients. Indeed, thorough metanalyses of additional retrospective and randomized control studies will help elucidate the role of immunosuppressants in predisposing COVID-19 patients to fungal co-infections.
This entry is adapted from the peer-reviewed paper 10.3390/jof7070545
References
- White P. L.; Dhillon; Alan Cordey; Harriet Hughes; Federica Faggian; Shuchita Soni; Manish Pandey; Harriet Whitaker; Alex May; Matt Morgan; et al. A National Strategy to Diagnose Coronavirus Disease 2019–Associated Invasive Fungal Disease in the Intensive Care Unit. Clinical Infectious Diseases 2020, x, ciaa1298, 10.1093/cid/ciaa1298.
- Jon Salmanton-García; Rosanne Sprute; Jannik Stemler; Michele Bartoletti; Damien Dupont; Maricela Valerio; Carolina García-Vidal; Iker Falces-Romero; Marina Machado; Sofía de la Villa; et al. COVID-19–Associated Pulmonary Aspergillosis, March–August 2020. Emerging Infectious Diseases 2021, 27, 1077-1086, 10.3201/eid2704.204895.
- Joseph Katz; Prevalence of candidiasis and oral candidiasis in COVID-19 patients: a cross-sectional pilot study from the patients' registry in a large health center. Quintessence international (Berlin, Germany : 1985) 2021, 0, 0, 10.3290/J.QI.B1491959.
- Marcio Nucci; Gloria Barreiros; Luiz Felipe Guimarães; Vitor A.S. Deriquehem; Anna Carla Castiñeiras; Simone A. Nouér; Increased incidence of candidemia in a tertiary care hospital with the COVID‐19 pandemic. Mycoses 2020, 64, 152-156, 10.1111/myc.13225.
- Obinna T Ezeokoli; Carolina H Pohl; Opportunistic pathogenic fungal co-infections are prevalent in critically ill COVID-19 patients: Are they risk factors for disease severity?. South African Medical Journal 2020, 110, 1081, 10.7196/samj.2020.v110i11.15248.
- Xiaojuan Zhu; Yiyue Ge; Tao Wu; Kangchen Zhao; Yin Chen; Bin Wu; Fengcai Zhu; Baoli Zhu; Lunbiao Cui; Co-infection with respiratory pathogens among COVID-2019 cases. Virus Research 2020, 285, 198005-198005, 10.1016/j.virusres.2020.198005.
- Amir Arastehfar; Tahmineh Shaban; Hossein Zarrinfar; Maryam Roudbary; Mona Ghazanfari; Mohammad-Taghi Hedayati; Alireza Sedaghat; Macit Ilkit; Mohammad Najafzadeh; David Perlin; et al. Candidemia among Iranian Patients with Severe COVID-19 Admitted to ICUs. Journal of Fungi 2021, 7, 280, 10.3390/jof7040280.
- Michele Bartoletti; Renato Pascale; Monica Cricca; Matteo Rinaldi; Angelo Maccaro; Linda Bussini; Giacomo Fornaro; Tommaso Tonetti; Giacinto Pizzilli; Eugenia Francalanci; et al. Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients With COVID-19: A Prospective Study. Clinical Infectious Diseases 2020, X, ciaa1065, 10.1093/cid/ciaa1065.
- Eelco F. J. Meijer; Anton S. M. Dofferhoff; Oscar Hoiting; Jacques F. Meis; COVID‐19–associated pulmonary aspergillosis: a prospective single‐center dual case series. Mycoses 2021, 64, 457-464, 10.1111/myc.13254.
- Andrea Mastrangelo; Bruno Nicolò Germinario; Marica Ferrante; Claudia Frangi; Raffaele Li Voti; Camilla Muccini; Marco Ripa; Diana Canetti; Barbara Castiglioni; Chiara Oltolini; et al. Candidemia in Coronavirus Disease 2019 (COVID-19) Patients: Incidence and Characteristics in a Prospective Cohort Compared With Historical Non–COVID-19 Controls. Clinical Infectious Diseases 2020, x, ciaa1594, 10.1093/cid/ciaa1594.
- Laura Magnasco; Malgorzata Mikulska; Daniele Giacobbe; Lucia Taramasso; Antonio Vena; Chiara Dentone; Silvia Dettori; Stefania Tutino; Laura Labate; Vincenzo Di Pilato; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris During the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship?. Microorganisms 2021, 9, 95, 10.3390/microorganisms9010095.
- Alejandro Suarez-De-La-Rica; Department of Anesthesiology and Surgical Critical Care. Hospital Universitario Marqués de Valdecilla. Santander. Spain.; Patricia Serrano; Rodrigo De-La-Oliva; Pedro Sánchez-Díaz; Pilar Molinero; Iker Falces-Romero; Carlos Ferrando; Jordi Rello; Emilio Maseda; et al. Secondary infections in mechanically ventilated patients with COVID-19: An overlooked matter?. Revista Española de Quimioterapia 2021, x, 1-7, 10.37201/req/031.2021.
- Anuradha Chowdhary; Bansidhar Tarai; Ashutosh Singh; Amit Sharma; Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerging Infectious Diseases 2020, 26, 2694-2696, 10.3201/eid2611.203504.
- Aia Mohamed; Tidi Hassan; Marta Trzos-Grzybowska; Jubil Thomas; Aidan Quinn; Maire O'Sullivan; Auveen Griffin; Thomas R. Rogers; Alida Fe Talento; Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: A lethal combination. Medical Mycology Case Reports 2021, 31, 11-14, 10.1016/j.mmcr.2020.06.005.
- Brunella Posteraro; Riccardo Torelli; Antonietta Vella; Paolo Maria Leone; Giulia De Angelis; Elena De Carolis; Giulio Ventura; Maurizio Sanguinetti; Massimo Fantoni; Pan-Echinocandin-Resistant Candida glabrata Bloodstream Infection Complicating COVID-19: A Fatal Case Report. null 2020, 6, x, 10.20944/preprints202008.0198.v1.
- Mucormycosis: The “Black Fungus” Maiming Covid Patients in India . BBC. Retrieved 2021-7-14
- Arunaloke Chakrabarti; Manpreet Dhaliwal; Epidemiology of Mucormycosis in India. Current Fungal Infection Reports 2013, 7, 287-292, 10.1007/s12281-013-0152-z.
- Amit Kumar Deb; Sandip Sarkar; Tanmay Gokhale; Sushmita Sana Choudhury; COVID-19 and orbital mucormycosis. Indian Journal of Ophthalmology 2021, 69, 1002-1004, 10.4103/ijo.ijo_3763_20.
- Christoph Zurl; Martin Hoenigl; Eduard Schulz; Stefan Hatzl; Gregor Gorkiewicz; Robert Krause; Philipp Eller; Juergen Prattes; Autopsy Proven Pulmonary Mucormycosis Due to Rhizopus microsporus in a Critically Ill COVID-19 Patient with Underlying Hematological Malignancy. Journal of Fungi 2021, 7, 88, 10.3390/jof7020088.
- Awadhesh Kumar Singh; Ritu Singh; Shashank R. Joshi; Anoop Misra; Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2021, 15, 102146-102146, 10.1016/j.dsx.2021.05.019.
- S Sharma; M Grover; S Bhargava; S Samdani; T Kataria; Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. The Journal of Laryngology & Otology 2021, 135, 442-447, 10.1017/s0022215121000992.
- Bindu Mulakavalupil; Charudatta Vaity; Shashank Joshi; Anoop Misra; Rahul Anil Pandit; Absence of Case of Mucormycosis (March 2020–May 2021) under strict protocol driven management care in a COVID-19 specific tertiary care intensive care unit. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2021, 15, 102169, 10.1016/j.dsx.2021.06.006.
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R.; Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia 2021, 186, 289–298, 10.1007/s11046-021-00528-2.
- Rosario Cultrera; Agostino Barozzi; Marco Libanore; Elisabetta Marangoni; Roberto Pora; Brunella Quarta; Savino Spadaro; Riccardo Ragazzi; Anna Marra; Daniela Segala; et al. Co-Infections in Critically Ill Patients with or without COVID-19: A Comparison of Clinical Microbial Culture Findings. International Journal of Environmental Research and Public Health 2021, 18, 4358, 10.3390/ijerph18084358.
- Amir Arastehfar; Agostinho Carvalho; Frank L. Van De Veerdonk; Jeffrey D. Jenks; Philipp Koehler; Robert Krause; Oliver A. Cornely; David S. Perlin; Cornelia Lass-Flörl; Martin Hoenigl; et al. COVID-19 Associated Pulmonary Aspergillosis (CAPA)—From Immunology to Treatment. Journal of Fungi 2020, 6, 91, 10.3390/jof6020091.
- Amir Arastehfar; Agostinho Carvalho; M. Hong Nguyen; Mohammad Taghi Hedayati; Mihai G. Netea; David S. Perlin; Martin Hoenigl; COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions?. Journal of Fungi 2020, 6, 211, 10.3390/jof6040211.
- Muluneh Worku; Friehiwot Girma; Candida auris: From Multidrug Resistance to Pan-Resistant Strains. Infection and Drug Resistance 2020, ume 13, 1287-1294, 10.2147/idr.s249864.
- Philipp Koehler; Matteo Bassetti; Arunaloke Chakrabarti; Sharon C A Chen; Arnaldo Lopes Colombo; Martin Hoenigl; Nikolay Klimko; Cornelia Lass-Flörl; Rita O Oladele; Donald C Vinh; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. The Lancet Infectious Diseases 2021, 21, e149-e162, 10.1016/s1473-3099(20)30847-1.
- Paul E. Verweij; Bart J. A. Rijnders; Roger J. M. Brüggemann; Elie Azoulay; Matteo Bassetti; Stijn Blot; Thierry Calandra; Cornelius J. Clancy; Oliver A. Cornely; Tom Chiller; et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Medicine 2020, 46, 1524-1535, 10.1007/s00134-020-06091-6.
- Lynn Rutsaert; Nicky Steinfort; Tine Van Hunsel; Peter Bomans; Reinout Naesens; Helena Mertes; Hilde Dits; Niels Van Regenmortel; COVID-19-associated invasive pulmonary aspergillosis. Annals of Intensive Care 2020, 10, 1-4, 10.1186/s13613-020-00686-4.
- Ceva Wicaksono Pitoyo; Dita Aditianingsih; Cleopas Martin Rumende; Risk factors for early invasive fungal disease in critically ill patients. Indian Journal of Critical Care Medicine 2016, 20, 633-639, 10.4103/0972-5229.194007.
- Amartya Chakraborti; Anand Jaiswal; Pushpendra Kumar Verma; Ritu Singhal; A prospective study of fungal colonization and invasive fungal disease in long-term mechanically ventilated patients in a respiratory intensive care unit. Indian Journal of Critical Care Medicine 2018, 22, 597-601, 10.4103/ijccm.ijccm_181_18.
- Sarah Dellière; Emmanuel Dudoignon; Sofiane Fodil; Sebastian Voicu; Magalie Collet; Pierre-Antoine Oillic; Maud Salmona; François Dépret; Théo Ghelfenstein-Ferreira; Benoit Plaud; et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clinical Microbiology and Infection 2021, 27, 790.e1-790.e5, 10.1016/j.cmi.2020.12.005.
- Vincent J. Venditto; Dalia Haydar; Ahmed Abdel-Latif; John C. Gensel; Michael I. Anstead; Michelle G. Pitts; Jarrod Creameans; Timothy J. Kopper; Chi Peng; David J. Feola; et al. Immunomodulatory Effects of Azithromycin Revisited: Potential Applications to COVID-19. Frontiers in Immunology 2021, 12, 574425, 10.3389/fimmu.2021.574425.
- Abdullah M.S. Al-Hatmi; Jalila Mohsin; Aisha Al-Huraizi; Faryal Khamis; COVID-19 associated invasive candidiasis. Journal of Infection 2021, 82, e45-e46, 10.1016/j.jinf.2020.08.005.
- COVID-19 Treatment Guidelines . National Institute of Health. Retrieved 2021-7-14
- Qing Ye; Bili Wang; Jianhua Mao; The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. Journal of Infection 2020, 80, 607-613, 10.1016/j.jinf.2020.03.037.
- Biying Hu; Shaoying Huang; Lianghong Yin; The cytokine storm and COVID‐19. Journal of Medical Virology 2020, 93, 250-256, 10.1002/jmv.26232.
- David C. Fajgenbaum; Carl H. June; Cytokine Storm. New England Journal of Medicine 2020, 383, 2255-2273, 10.1056/nejmra2026131.
- The RECOVERY Collaborative Group; Dexamethasone in Hospitalized Patients with Covid-19. New England Journal of Medicine 2021, 384, 693-704, 10.1056/nejmoa2021436.
- Russell Lewis; Dimitrios P. Kontoyiannis; Invasive aspergillosis in glucocorticoid-treated patients. Medical Mycology 2009, 47, S271-S281, 10.1080/13693780802227159.
- Michail S Lionakis; Dimitrios P Kontoyiannis; Glucocorticoids and invasive fungal infections. The Lancet 2003, 362, 1828-1838, 10.1016/s0140-6736(03)14904-5.
- Agnes E. Coutinho; Karen E. Chapman; The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular and Cellular Endocrinology 2011, 335, 2-13, 10.1016/j.mce.2010.04.005.
- Emmanuel Oppong; Andrew Cato; Effects of Glucocorticoids in the Immune System. Advances in Experimental Medicine and Biology 2015, 872, 217-233, 10.1007/978-1-4939-2895-8_9.
- Darius Armstrong-James; Jonathan Youngs; Tihana Bicanic; Alireza Abdolrasouli; David W. Denning; Elizabeth Johnson; Varun Mehra; Tony Pagliuca; Brijesh Patel; Johanna Rhodes; et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. European Respiratory Journal 2020, 56, 2002554, 10.1183/13993003.02554-2020.
- J Peter Donnelly; Sharon C Chen; Carol A Kauffman; William J Steinbach; John W Baddley; Paul Verweij; Cornelius J Clancy; John R Wingard; Shawn R Lockhart; Andreas H Groll; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clinical Infectious Diseases 2019, 71, 1367-1376, 10.1093/cid/ciz1008.
- Nosheen Nasir; Fazal Rehman; Syed Furrukh Omair; Risk factors for bacterial infections in patients with moderate to severe COVID‐19: A case‐control study. Journal of Medical Virology 2021, 93, 4564-4569, 10.1002/jmv.27000.
- Obinna Ezeokoli; Onele Gcilitshana; Carolina Pohl; Risk Factors for Fungal Co-Infections in Critically Ill COVID-19 Patients, with a Focus on Immunosuppressants. Journal of Fungi 2021, 7, 545, 10.3390/jof7070545.
- Cezar V. W. Riche; Renato Cassol; Alessandro C. Pasqualotto; Is the Frequency of Candidemia Increasing in COVID-19 Patients Receiving Corticosteroids?. Journal of Fungi 2020, 6, 286, 10.3390/jof6040286.
- Shruti Gupta; Wei Wang; Salim S. Hayek; Lili Chan; Kusum S. Mathews; Michal L. Melamed; Samantha K. Brenner; Amanda Leonberg-Yoo; Edward J. Schenck; Jared Radbel; et al. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Internal Medicine 2021, 181, 41, 10.1001/jamainternmed.2020.6252.
- Lucas Kimmig; David Wu; Matthew Gold; Natasha N. Pettit; David Pitrak; Jeffrey Mueller; Aliya N. Husain; Ece A. Mutlu; Gokhan Mutlu; IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Frontiers in Medicine 2020, 7, 583897, 10.3389/fmed.2020.583897.
- Kam Sing Ho; Bharat Narasimhan; Larry Difabrizio; Linda Rogers; Sonali Bose; Li Li; Roger Chen; Jacqueline Sheehan; Maan Ajwad El-Halabi; Kimberly Sarosky; et al. Impact of corticosteroids in hospitalised COVID-19 patients. BMJ Open Respiratory Research 2021, 8, e000766, 10.1136/bmjresp-2020-000766.
- João de Almeida; Elaine Francisco; Ferry Hagen; Igor Brandão; Felicidade Pereira; Pedro Presta Dias; Magda De Miranda Costa; Regiane De Souza Jordão; Theun de Groot; Arnaldo Colombo; et al. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. Journal of Fungi 2021, 7, 220, 10.3390/jof7030220.
- Spinello Antinori; Laura Milazzo; Salvatore Sollima; Massimo Galli; Mario Corbellino; Candidemia and invasive candidiasis in adults: A narrative review. European Journal of Internal Medicine 2016, 34, 21-28, 10.1016/j.ejim.2016.06.029.
