Skin aging is associated with the accumulation of senescent cells and is related to many pathological changes, including decreased protection against pathogens, increased susceptibility to irritation, delayed wound healing, and increased cancer susceptibility. Senescent cells secrete a specific set of pro-inflammatory mediators, referred to as a senescence-associated secretory phenotype (SASP), which can cause profound changes in tissue structure and function. Thus, drugs that selectively eliminate senescent cells (senolytics) or neutralize SASP (senostatics) represent an attractive therapeutic strategy for age-associated skin deterioration. There is growing evidence that plant-derived compounds (flavonoids) can slow down or even prevent aging-associated deterioration of skin appearance and function by targeting cellular pathways crucial for regulating cellular senescence and SASP. This review summarizes the senostatic and senolytic potential of flavonoids in the context of preventing skin aging.
- senescent cells
- senescence-associated secretory phenotype (SASP)
- flavonoids
- senolytics
- senostatics
1. Introduction
Besides being an economic and social problem, aging is predominantly a medical issue. Thus, there is an increasing need to understand the mechanisms underlying this highly complex process[1], which inevitably leads to impaired body homeostasis and function, an increased risk of complex diseases, and, finally, death.
Cellular senescence contributes to age-related tissue and organ dysfunction and diseases through mechanisms that perturb stem cell niches, induce aberrant cell differentiation, disrupt the extracellular matrix, stimulate tissue inflammation, and induce senescence in neighboring cells [2][3]. It is believed that the accumulation of senescent cells in tissues contributes to the impairment of their homeostasis and increases the risk of many age-related diseases[4]. Therefore, eliminating senescent cells or neutralizing SASP components may provide beneficial effects not only for the affected tissue but also the whole organism. Drugs that selectively eliminate senescent cells (senolytics) or neutralize SASP (senostatics) represent an attractive therapeutic strategy for delaying aging and age-related diseases[5].
Skin aging is associated with an increasing number of senescent cells and is related to many pathological changes, including decreased protection against pathogens, increased susceptibility to irritation, delayed wound healing, and increased cancer susceptibility[6]. Therefore, therapies that reduce senescent cell numbers or block SASP may be an effective treatment for aging-associated skin deterioration[7]. The senolytic and senostatic activities of several drugs (e.g., metformin and rapamycin) have already been demonstrated in preliminary clinical trials[8][9]. However, in vitro and in vivo data show that different flavonoids have similar properties; therefore, they can be considered a therapeutic option for skin aging prevention and treatment.
2. Therapeutic Strategies Targeting Skin Senescence
Due to the harmful effects of senescent cells and SASP components on many tissues, strategies aimed at selective induction of senescent cell death or inhibiting SASP without affecting the selective induction of death of surrounding cells are currently being investigated[10]. Removal of senescent cells from aging tissues is considered a promising anti-aging therapy. However, under certain circumstances, such skin cells can also play a positive role[11]. Therefore, SASP modification and maintaining the beneficial features of cell senescence seem to be a more rational therapeutic approach than senescent cell removal.
Complex signaling pathways control SASP production. Nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) is a crucial transcription factor for SASP induction. However, the DNA damage response (DDR), p38 mitogen-activated protein kinase (MAPK), CCAAT/enhancer-binding protein b (C/EBPb), mechanistic target of rapamycin (mTOR), phosphoinositide-3-kinase (PI3K), Janus kinase/signal transducer and activator of transcription (JAK/STAT), protein kinase D1, and several other factors are also involved in regulating SASP production by senescent cells[12].
Different drugs specifically block the signals associated with senescent cell secretion. For example, glucocorticosteroids can reduce SASP secretion and inflammation induced by senescent cells and SASP due to their ability to decrease the transcriptional activity of NF-κB However, several adverse side effects of glucocorticoid treatment (e.g., skin thinning and impaired wound healing) limit their application as skin senolytics[13]. There is growing evidence that flavonoids can prevent skin from aging by targeting cellular pathways crucial for regulating cellular senescence and SASP production.
3. Flavonoids as a Senostatic and Senolytic Strategy
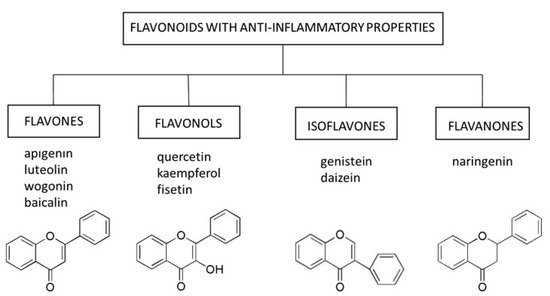
3.1. Flavones
Flavones occur in a wide variety of fruits, vegetables, and cereal grains in the form of glycosides. As with other flavonoid glycosides in foods, flavones must be hydrolyzed to aglycones to be absorbed. They are then metabolized to glucuronidated or sulfated forms before reaching systemic circulation. The main flavones in the diet are apigenin and luteolin; however, some other compounds (e.g., baicalin and wogonin) are also worth mentioning[21].
3.1.1. Apigenin
Apigenin, a flavone present in select fruits, vegetables, and herbs, can induce apoptosis and inhibit proliferation and angiogenesis in several cancer cell lines[22]. The anti-cancer activities of apigenin result from its ability to interact with the PI3K/protein kinase B (ERK)/mTOR, JAK/STAT, NF-κB, MAPK, and Wnt/β-catenin pathways[23]. Interference with mTOR signaling is a dominant mechanism by which apigenin inhibits skin cancer development and progression[24]. Moreover, apigenin has antioxidant and anti-inflammatory properties and can restore the proper function of the skin (e.g., DNA repair and viability of human keratinocytes and dermal fibroblasts) after damage caused by exposure to UVA and UVB radiation[25][26][27]. The molecular mechanisms underlying these phenomena involve the ability of apigenin to inhibit the expression of cyclooxygenase-2 (COX-2) and the NF-κB pathway, which controls the inflammation caused by UVA and UVB radiation[22]. The interaction between apigenin and the NF-κB pathway also seems to be a key mechanism for reducing the secretion of several SASP factors (e.g., IL-6 and IL-8) in human fibroblasts induced to undergo senescence by bleomycin[18]. Moreover, topical administration of apigenin to mice exposed to UVB radiation reduced cutaneous inflammation by inducing thrombospondin 1 (TSP-1) expression and repressing IL-6 and IL-12 levels and inflammatory infiltrates[28].
3.1.2. Baicalin
3.1.3. Luteolin
3.1.4. Wogonin
3.2. Flavonols
3.2.1. Quercetin
3.2.2. Kaempferol
3.2.3. Fisetin
3.3. Isoflavones
3.3.1. Daidzein and Genistein
3.4. Flavanones
4. Summary and Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms22136814
References
- Van Deursen, J.M.; The role of senescent cells in ageing. Nature 2014, 509, 439–446, 10.1038/nature13193.
- Muñoz-Espín, D.; Serrano, M.; Cellular senescence: From physiology to pathology.. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496, 10.1038/nrm3823.
- Jesus, B.B.; De Blasco, M.A.; Europe PMC Funders Group Assessing Cell and Organ Senescence Biomarkers.. Circ. Res. 2016, 111, 97–109, 10.1161/CIRCRESAHA.111.247866..
- Kaur, J.; Farr, J.N.; Cellular senescence in age-related disorders. . Transl. Res. 2020, 226, 96–104., .
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.E.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs.. Aging Cell 2015, 14, 644–658., .
- Laberge, R.-M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation.. Nat. Cell Biol. 2015, 17, 1049–1061, .
- Kirkland, J.L.; Tchkonia, T.; Cellular Senescence: A Translational Perspective. . EBioMedicine 2017, 21, 21–28., .
- De Kreutzenberg, S.V.; Ceolotto, G.; Cattelan, A.; Pagnin, E.; Mazzucato, M.; Garagnani, P.; Borelli, V.; Bacalini, M.G.; Franceschi, C.; Fadini, G.P.; et al. Metformin improves putative longevity effectors in peripheral mononuclear cells from subjects with prediabetes. A randomized controlled trial. . Nutr. Metab. Cardiovasc. Dis. 2015, 25, 686–693, .
- Kraig, E.; Linehan, L.A.; Liang, H.; Romo, T.Q.; Liu, Q.; Wu, Y.; Benavides, A.D.; Curiel, T.J.; Javors, M.A.; Musi, N.; et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. . Exp. Gerontol. 2018, 105, 53–69., .
- Velarde, M.; De Maria, M.; Targeting Senescent Cells: Possible Implications for Delaying Skin Aging: A Mini-Review. . Gerontology 2016, 62, 513–518, .
- Regulski, M.J.; Cellular Senescence: What, Why and How. . Wounds 2017, 29, 168–174, .
- Salminen, A.; Kauppinen, A.; Kaarniranta, K.; Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). . Cell. Signal. 2012, 24, 835–845, .
- Abraham, A.; Roga, G.; Topical steroid-damaged skin.. Indian J. Dermatol. 2014, 59, 456–459, .
- Kumar, S.; Pandey, A.K.; Chemistry and Biological Activities of Flavonoids: An Overview. . Sci. World J. 2013, 2013, 162750., .
- Panche, A.N.; Diwan, A.D.; Chandra, S.R.; Flavonoids: An overview.. J. Nutr. Sci. 2016, 5, e47, .
- Kuryłowicz, A.; The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review.. Int. J. Mol. Sci. 2020, 22, 218, .
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S.; Flavonoids in modulation of cell survival signalling pathways.. Genes Nutr 2014, 9, 1–9, .
- Lim, H.; Park, H.; Kim, H.P.; Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. . Biochem. Pharmacol. 2015, 96, 337–348, .
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B.; Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases.. Int. J. Mol. Sci. 2016, 17, 921, .
- Laura, V.; Mattia, F.; Roberta, G.; Federico, I.; Emi, D.; Chiara, T.; Luca, B.; Elena, C.; Potential of Curcumin in Skin Disorders. . Nutrients 2019, 11, 2169, .
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J.; Flavones: Food Sources, Bioavailability. . Adv Nutr 2017, 8, 423–435, .
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin.. Int. J. Mol. Sci. 2019, 20, 1305, .
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B.; Rationalizing the therapeutic potential of apigenin against cancer.. Life Sci. 2021, 167, 118814, .
- Bridgeman, B.B.; Wang, P.; Ye, B.; Pelling, J.C.; Volpert, O.V.; Tong, X.; Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skin cancer prevention.. Cell. Signal. 2016, 28, 460–468, .
- Tong, X.; Mirzoeva, S.; Veliceasa, D.; Bridgeman, B.B.; Fitchev, P.; Cornwell, M.L.; Crawford, S.E.; Pelling, J.C.; Volpert, O.V.; Chemopreventive apigenin controls UVB-induced cutaneous proliferation and angiogenesis through HuR and thrombospondin-1. . Oncotarget 2014, 5, 11413–11427, .
- Choi, S.; Youn, J.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.-S.; Kwon, S.; Youn, H.J.; et al. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo.. Int. J. Mol. Med. 2016, 38, 627–634, .
- Britto, S.M.; Shanthakumari, D.; Agilan, B.; Radhiga, T.; Kanimozhi, G.; Prasad, N.R.; Apigenin prevents ultraviolet-B radiation induced cyclobutane pyrimidine dimers formation in human dermal fibroblasts.. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 821, 28–35, .
- Mirzoeva, S.; Tong, X.; Bridgeman, B.B.; Plebanek, M.P.; Volpert, O.V.; Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. . Neoplasia 2018, 20, 930–942, .
- Perrott, K.M.; Wiley, C.D.; Desprez, P.-Y.; Campisi, J.; Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells.. GeroScience 2017, 39, 161–173, .
- Das, S.; Das, J.; Paul, A.; Samadder, A.; Khuda-Bukhsh, A.R.; Apigenin, a Bioactive Flavonoid from Lycopodium clavatum, Stimulates Nucleotide Excision Repair Genes to Protect Skin Keratinocytes from Ultraviolet B-Induced Reactive Oxygen Species and DNA Damage.. J. Acupunct. Meridian Stud. 2013, 6, 252–262, .
- Zhang, Y.; Wang, J.; Cheng, X.; Yi, B.; Zhang, X.; Li, Q.; Apigenin induces dermal collagen synthesis via smad2/3 signaling pathway. . Eur. J. Histochem 2015, 59, 2467, .
- Ve, A.S.G.; Apigenin as an Anti-Aging Skin Treatment.. J. Clin. Cosmet. Dermatol. 2018, 2, 1–8, .
- Bing-Rong, Z.; Song-Liang, J.; Xiao, E.C.; Xiang-Fei, L.; Bao-Xiang, C.; Jie, G.; Dan, L.; Protective effect of the Baicalin against DNA damage induced by ultraviolet B irradiation to mouse epidermis. . Photodermatol. Photoimmunol. Photomed. 2008, 24, 175–182, .
- Kim, D.H.; Kim, H.K.; Park, S.; Kim, J.Y.; Zou, Y.; Cho, K.H.; Kim, Y.S.; Kim, D.H.; Yu, B.P.; Choi, J.S.; et al. Short-term feeding of baicalin inhibits age-associated NF-κB activation. . Mech. Ageing Dev. 2006, 127, 719–725, .
- Zhang, J.-A.; Yin, Z.; Ma, L.-W.; Yin, Z.-Q.; Hu, Y.-Y.; Xu, Y.; Wu, D.; Permatasari, F.; Luo, D.; Zhou, B.-R.; et al. The Protective Effect of Baicalin against UVB Irradiation Induced Photoaging: An In Vitro and In Vivo Study.. PLoS ONE 2014, 9, e99703, .
- Wang, S.-C.; Chen, S.-F.; Lee, Y.-M.; Chuang, C.-L.; Bau, D.-T.; Lin, S.-S.; Baicalin scavenges reactive oxygen species and protects human keratinocytes against UVC-induced cytotoxicity.. In Vivo 2013, 27, 707–714, .
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U.; Luteolin as a modulator of skin aging and inflammation.. BioFactors 2020, 47, 170–180, .
- Wölfle, U.; Haarhaus, B.; Schempp, C.M.; The Photoprotective and Antioxidative Properties of Luteolin are Synergistically Augmented by Tocopherol and Ubiquinone.. Planta Med. 2013, 79, 963–965, .
- Wölfle, U.; Heinemann, A.; Esser, P.R.; Haarhaus, B.; Martin, S.F.; Schempp, C.M.; Luteolin Prevents Solar Radiation-Induced Matrix Metalloproteinase-1 Activation in Human Fibroblasts: A Role for p38 Mitogen-Activated Protein Kinase and Interleukin-20 Released from Keratinocytes.. Rejuvenat. Res. 2012, 15, 466–475, .
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C.; Differential Regulation of Hyaluronan Metabolism in the Epidermal and Dermal Compartments of Human Skin by UVB Irradiation.. J. Investig. Dermatol. 2007, 127, 687–697, .
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G.; The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells.. J. Dermatol. Sci. 2011, 61, 23–31, .
- Hwang, Y.P.; Choi, J.H.; Kim, H.G.; Choi, J.M.; Hwang, S.K.; Chung, Y.C.; Jeong, H.G.; Cultivated ginseng suppresses ultraviolet B–induced collagenase activation via mitogen-activated protein kinases and nuclear factor κB/activator protein-1–dependent signaling in human dermal fibroblasts. . Nutr. Res. 2012, 32, 428–438, .
- Yang, D.; Guo, Q.; Liang, Y.; Zhao, Y.; Tian, X.; Ye, Y.; Tian, J.; Wu, T.; Lu, N.; Wogonin induces cellular senescence in breast cancer via suppressing TXNRD2 expression. . Arch. Toxicol. 2020, 94, 3433–3447, .
- Chi, Y.; Kim, H.; Suppression of cyclooxygenase-2 expression of skin fibroblasts by wogonin, a plant flavone from Scutellaria radix.. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 59–66, .
- Wang, Y.-S.; Cho, J.-G.; Hwang, E.-S.; Yang, J.-E.; Gao, W.; Fang, M.-Z.; Zheng, S.-D.; Yi, T.-H.; Enhancement of Protective Effects of Radix Scutellariae on UVB-induced Photo Damage in Human HaCaT Keratinocytes.. Appl. Biochem. Biotechnol. 2018, 184, 1073–1093, .
- Park, B.K.; Heo, M.Y.; Park, H.; Kim, H.P.; Inhibition of TPA-induced cyclooxygenase-2 expression and skin inflammation in mice by wogonin, a plant flavone from Scutellaria radix. . Eur. J. Pharmacol. 2001, 425, 153–157, .
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P.; Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. . Biochem. Pharmacol. 2003, 66, 1271–1278, .
- Company, A.; Lloret-Fillol, J.; Costas, M. . Small Molecule Models for Nonporphyrinic Iron and Manganese Oxygenases; Company, A.; Lloret-Fillol, J.; Costas, M. , Eds.; Elsevier BV: Amsterdam, The Nethrelands, , 2013; pp. 487–564..
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.-G.; Byun, S.; Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin.. Int. J. Mol. Sci. 2019, 20, 5262, .
- Wermuth, P.; Addya, S.; Jimenez, S.A.; Effect of Protein Kinase C delta (PKC-δ) Inhibition on the Transcriptome of Normal and Systemic Sclerosis Human Dermal Fibroblasts In Vitro. . PLoS ONE 2011, 6, e27110, .
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Olszowka, J.; Kulpa-Greszta, M.; Litwinienko, G.; Tomaszewska, A.; Wnuk, M.; Pazik, R.; AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts. . Redox Biol 2020, 28, 101337, .
- Vicentini, F.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.; Fisher, G.J.; Xu, Y.; Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway. . J. Dermatol. Sci. 2011, 61, 162–168, .
- García-Mediavilla, M.V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J.; The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. . Eur. J. Pharmacol. 2007, 557, 221–229, .
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. . EBioMedicine 2018, 36, 18–28, .
- Seo, S.-H.; Jeong, G.-S.; Fisetin inhibits TNF-α-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. . Int. Immunopharmacol 2015, 29, 246–253, .
- Chiang, H.-M.; Chan, S.-Y.; Chu, Y.; Wen, K.-C.; Fisetin Ameliorated Photodamage by Suppressing the Mitogen-Activated Protein Kinase/Matrix Metalloproteinase Pathway and Nuclear Factor-κB Pathways.. J. Agric. Food Chem. 2015, 63, 4551–4560, .
- Wu, P.-Y.; Lyu, J.-L.; Liu, Y.-J.; Chien, T.-Y.; Hsu, H.-C.; Wen, K.-C.; Chiang, H.-M.; Fisetin Regulates Nrf2 Expression and the Inflammation-Related Signaling Pathway to Prevent UVB-Induced Skin Damage in Hairless Mice. . Int. J. Mol. Sci. 2017, 18, 2118, .
- Alleviation by Fisetin of Frailty, Inflammation, and Related Measures in Older Adults—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03675724
- Barnes, S.; The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and their Food Products.. Lymphat. Res. Biol. 2010, 8, 89–98, .
- Kim, S.-Y.; Kim, S.-J.; Lee, J.-Y.; Kim, W.-G.; Park, W.-S.; Sim, Y.-C.; Lee, S.-J.; Protective effects of dietary soy isoflavones against UV-induced skin-aging in hairless mouse model.. J. Am. Coll. Nutr. 2004, 23, 157–162, .
- Zhao, D.; Shi, Y.; Dang, Y.; Zhai, Y.; Ye, X.; Daidzein stimulates collagen synthesis by activating the TGF-β/smad signal pathway. . Australas. J. Dermatol. 2014, 56, e7–e14, .
- Oh, H.-J.; Kang, Y.-G.; Na, T.-Y.; Kim, H.-J.; Park, J.S.; Cho, W.-J.; Lee, M.-O.; Identification of daidzein as a ligand of retinoic acid receptor that suppresses expression of matrix metalloproteinase-9 in HaCaT cells. . Mol. Cell. Endocrinol. 2013, 376, 107–113, .
- Widyarini, S.; Spinks, N.; Husband, A.J.; Reeve, V.E.; Isoflavonoid Compounds from Red Clover (Trifolium pratense) Protect from Inflammation and Immune Suppression Induced by UV Radiation.. Photochem. Photobiol. 2001, 74, 465, .
- Reeve, V.E.; Widyarini, S.; Domanski, D.; Chew, E.; Barnes, K.; Protection Against Photoaging in the Hairless Mouse by the Isoflavone Equol.. Photochem. Photobiol. 2005, 81, 1548–1553, .
- Polito, F.; Marini, H.R.; Bitto, A.; Irrera, N.; Vaccaro, M.; Adamo, E.B.; Micali, A.; Squadrito, F.; Minutoli, L.; Altavilla, D.; et al. Genistein aglycone, a soy-derived isoflavone, improves skin changes induced by ovariectomy in rats. . Br. J. Pharmacol. 2011, 165, 994–1005, .
- Na Wang, Y.; Wu, W.; Chen, H.C.; Fang, H.; Genistein protects against UVB-induced senescence-like characteristics in human dermal fibroblast by p66Shc down-regulation.. J. Dermatol. Sci. 2010, 58, 19–27, .
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Monfrecola, G.; Bevilacqua, M.A.; Synergic Effect of Genistein and Daidzein on UVB-Induced DNA Damage: An Effective Photoprotective Combination.. J. Biomed. Biotechnol. 2011, 2011, 1–8, .
- Miyazaki, K.; Hanamizu, T.; Iizuka, R.; Chiba, K.; Genistein and Daidzein Stimulate Hyaluronic Acid Production in Transformed Human Keratinocyte Culture and Hairless Mouse Skin. . Skin. Pharmacol. Physiol. 2002, 15, 175–183, .
- Izumi, T.; Saito, M.; Obata, A.; Arii, M.; Yamaguchi, H.; Matsuyama, A.; Oral intake of soy isoflavone aglycone improves the aged skin of adult women.. J. Nutr. Sci. Vitaminol. 2007, 53, 57–62, .
- Moraes, A.B.; Haidar, M.A.; Soares, J.M.; Simões, M.J.; Baracat, E.C.; Patriarca, M.T.; The effects of topical isoflavones on postmenopausal skin: Double-blind and randomized clinical trial of efficacy. . Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 188–192, .
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.-E.; Wani, G.; Patnaik, S.; Zhao, Q.; Arafa, E.-S.; Barakat, B.; Mir, S.N.; Wani, A.A.; et al. Naringenin Protects HaCaT Human Keratinocytes Against UVB-induced Apoptosis and Enhances the Removal of Cyclobutane Pyrimidine Dimers from the Genome.. Photochem. Photobiol. 2007, 84, 307–316, .
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R.; et al. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. . J. Nat. Prod. 2015, 78, 1647–1655, .
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.V.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; et al. Topical Formulation Containing Naringenin: Efficacy against Ultraviolet B Irradiation-Induced Skin Inflammation and Oxidative Stress in Mice.. PLoS ONE 2016, 11, e0146296, .
- Lim, K.H.; Kim, G.R.; Inhibitory effect of naringenin on LPS-induced skin senescence by SIRT1 regulation in HDFs. . Biomed. Dermatol. 2018, 2, 26, .
