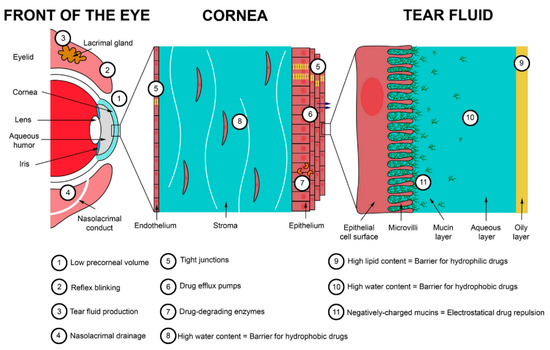
3. Chitosan Coatings
CS is a biocompatible polymer that, among others, can be used to enhance the mucoadhesion of drug formulations to tissues. Furthermore, the thiolation of CS can improve the penetration of NPs or their content through the tight junctions of mucus layers. Numerous studies have been conducted on the use of CS or its derivatives as coatings in order to deliver drugs encapsulated in liposomes, solid lipid matrixes, or NPs to the eye.
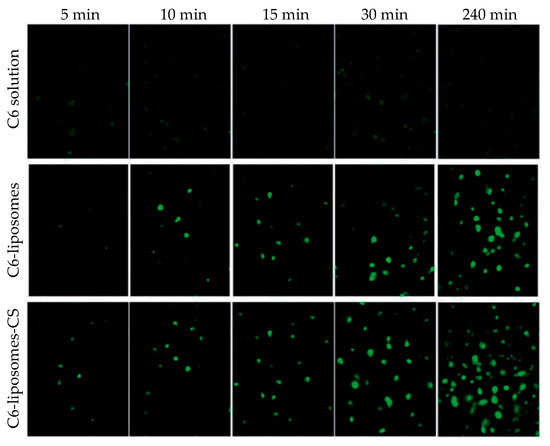
Li et al. encapsulated triamcinolone acetonide, which is an intermediate-acting glucocorticoid applied in inflammatory, edematous, and angiogenic ocular diseases, in liposomes, and the resulting NPs were further coated with CS [
294]. The coating of NPs resulted in a slight increase of the size, i.e., from 108 to 135 nm, while the ζ-potential was inverted from −10 to +18 mV. Coated formulations were stable for a 60-day period at 4 °C, although a slight decrease (about 5%) in the drug entrapment efficiency was observed. Concerning the release profile of the drug, CS coating enhanced drug release compared to the uncoated NPs. Cellular uptake was enhanced by the CS coating, as evidenced by fluorescence microscopy (). In vivo studies, performed on C57BL/6 mice, showed that the CS coating of liposomes led to a more efficient ocular delivery of triamcinolone acetonide to the posterior segment of the eye than eye drop formulation. Ocular toxicity tests showed that no toxicity was observed for either coated or uncoated liposomic NPs. The efficiency of these CS-coated liposomes in the treatment of retinal edema was further studied [
295]. Experiments that were conducted in vivo using rat models concluded that CS-coated liposomes could deliver the drug into the retina of the eye. Furthermore, the formulation was found to effectively relieve retinal edema, caused by laser, without showing toxicity.
Figure 20. Cellular uptake of triamcinolone acetonide/coumarin 6 liposomes (C6-liposomes) and chitosan-coated triamcinolone acetonide/coumarin 6 liposomes (C6-liposomes-CS) by corneal epithelial cells at different times. Reprinted from ref. [
294]. Rights managed by Taylor and Francis.
Tan et al. developed CS-coated liposomes for the ocular delivery of timolol maleate [
296]. The drug-loaded liposomes were prepared using an ammonium sulfate gradient coupled with a pH gradient. The CS coating was applied simply by stirring the liposomes in a CS solution. Increasing the CS concentration afforded bigger particles with an initially increasing ζ-potential. Compared to timolol maleate eye drop formulation, in vitro release from the coated liposomes was extended over 12 h with a lower initial burst release. Transcorneal permeation was significantly enhanced, and ocular retention was improved as well. As a result, the IOP lowering efficiency was improved. Khalil et al. also prepared CS-coated liposomes for the nanoencapsulation of triamcinolone acetonide for posterior eye delivery [
297]. The research team succeeded in preparing uncoated NPs of 18 nm in size while after coating with CS in concentrations of 0.1%, 0.2% and 0.3%
w/v, their size increased to 100, 170, and 176 nm. In vivo studies were conducted in a rat model showing that after 15 days, the drug was present in the posterior chamber of the eye, which is a conclusion that is in accordance with the studies conducted by Li et al. [
294] and Cheng et al. [
295].
Chen et al. prepared CS-coated deformable liposomes for the ocular delivery of flurbiprofen [
298]. The prepared NPs of deformable liposomes containing the drug were further coated by CS (M
w 50 kDa, degree of deacetylation: 95%) in three different concentrations (0.1%, 0.2%, and 0.4%
w/v). It was found that the coated NPs were larger than the uncoated ones, and the ζ-potential changed from negative to positive. A corneal penetration study showed that deformable liposomes had a 1.5-fold increased penetration compared to non-deformable ones, while their penetration ability was further enhanced by the CS coating. Indeed, CS can improve the permeability of the cornea by opening the tight junctions among corneal epithelial cells or by intracellular routes (
vide supra). A notable observation was that the penetration rate was different for the different formulations: all uncoated formulations showed a constant penetration rate, while the coated ones showed a reduced rate after 160 min. The cumulative penetration remained higher for the coated particles. In vivo pre-corneal retention showed that the CS coating significantly prolonged the residence time of deformable liposomes in the cornea and improved the drug bioavailability by increasing its transport time across the cornea.
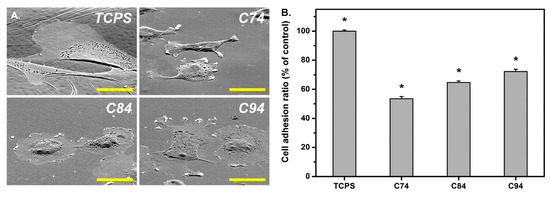
Sun et al. studied for the first time the influence of the deacetylation degree of CS on corneal keratocyte adhesion, spreading, morphology, and integrin gene expression when used as a coating in pharmaceutical formulations [
299]. The authors used CS with a molecular weight of 400 kDa, having three different degrees of deacetylation (74.1 ± 0.5%, 84.4 ± 0.7%, and 94.2 ± 0.5%). Initially, CS was 74% deacetylated. Further deacetylation was conducted via CS treatment with 60% (
w/v) NaOH solution for 1 h once and for 1.5 h twice at 100 °C under a nitrogen atmosphere to obtain deacetylation degrees of 84% and 94%, respectively. FTIR and gel permeation chromatography showed that deacetylation did not affect the initial molecular weight. Crystallinity increased as the degree of deacetylation increased, which was due to the absence of acetyl groups. An important issue revealed by the study is that the degree of deacetylation affected cell adhesion, i.e., an increased deacetylation degree resulted in enhanced cell adhesion (), which was probably due to the high stiffness and crystallinity of the biopolymers. It was suggested that the degree of deacetylation of CS coatings greatly affected cell adhesion-related phenomena and cell–substrate crosstalk during corneal keratocyte cultivation.
Figure 21. (
A) Rabbit corneal keratocyte morphology on tissue culture polystyrene (TCPS) and chitosan substrates with various deacetylation degrees: 74% (C74), 84% (C84), and 94% (C94). Scale bar represents 30 μm. (
B) Quantitative measurement of cell adhesion ratio for TCPS and various chitosan samples (* P b 0.05;
n = 4). Data in the experimental groups are percentages relative to those of the TCPS groups. Reprinted from ref. [
299]. Copyright 2016, with permission from Elsevier B.V.
Eid et al. studied the influence of pegylation and CS coating of ofloxacin lipid NPs [
300]. Both the pegylation of lipid NPs and CS coating resulted in bigger NPs. Their shape was nearly spherical, with a smooth surface. Both pegylation and the CS coating resulted in a two- to threefold increase in the amount of ofloxacin that could be delivered to ocular fluids and tissues compared to commercial Oflox
@ drops. Ban et al. reported the CS coating of dexamethasone-containing lipid NPs [
301]. As a result of CS coating, the ζ-potential shifted from negative to positive values, and a higher permeation was observed. The lipid NPs exhibited a higher bioavailability compared to dexamethasone aqueous solution. Gelfuso et al. studied the use of iontophoresis as a method to increase voriconazole release, leading to an enhanced initial burst effect [
302]. In brief, three different formulations were used: a cyclodextrin inclusion complex, a liposomal NP, and a CS-coated liposomal NP. After applying iontophoresis for 10 min, voriconazole penetration into the cornea was enhanced in all three formulations, with uncoated liposomal NPs showing the lowest concentration of the drug.
Seyfoddin et al. used a blend of different esters of behenic acid with glycerol (commercially available as Compritol
® 888 ATO) as a lipid carrier for the nanoencapsulation of acyclovir, which is a drug that is used in the therapy of herpes keratitis: the most common infectious cause of blindness [
303]. NPs were formed via the “hot microemulsion technique” and coated thereafter with CS, via their dispersion in solutions of different CS concentrations. The coating was achieved due to electrostatic interactions between the negatively charged lipid carrier and the positively charged CS. The authors studied the influence of the lyophilization process on the resulting NPs. It was observed that NPs tended to aggregate after freeze drying, resulting in higher sizes reaching the micro-scale range. Freshly prepared coated NPs showed small particle sizes between 323 and 468 nm; the size increased with increasing CS concentration. The ζ-potential was also proportional to the increase of CS concentration, starting from about −26 mV for uncoated NPs to about +28 mV when 1%
w/v CS was used. No significant difference was observed between CS concentrations of 0.5% and 1%
w/v. Drug release was studied in PBS pH 7.4. It was found that coating with CS led to a reduction of about 25% in acyclovir release rate compared to uncoated NPs, regardless of the CS concentration used for coating. The cellular uptake of NPs was enhanced by CS coating while the concentration of fluorescein increased with CS concentration and exposure time in the cytoplasm of the epithelial cells. In ex vivo penetration studies, conducted in bovine eyes, the coated NPs had enhanced properties, with the coating obtained from the 0.5%
w/v CS concentration showing the best results. The quantification of acyclovir in cellular uptake was higher for the coated NPs.
Selvaraj et al. designed CS-coated NLCs for the ocular delivery of itraconazole [
304]. The CS coating delayed itraconazole release due to the formation of a hydrophilic matrix around the lipid carriers, and ex vivo corneal permeability was significantly enhanced. In the antineovascularization study, CS-coated NLCs demonstrated a high reduction in neovascularization, which was attributed to low pre-corneal drainage, due to the higher mocoadhesivity and higher corneal permeation. Wang et al. used glyceryl monostearate as the solid lipid in the NP formulation of methazolamide used in glaucoma treatment [
305]. Phospholipid was used as the surfactant in a modified oil-in-water emulsification technique, while CS was used for the coating of NPs. The results are in accordance with other studies; i.e., a smooth surface increased the particle size compared to uncoated NPs and prolonged drug release. In vivo results showed that coated NPs successfully delivered methazolamide in rabbit eyes, showing a marked decrease in IOP and a better sustainability than the uncoated ones.
Dukovski et al. investigated the performance of a cationic nanoemulsion containing ibuprofen for the treatment of dry eye disease, in order to resolve ibuprofen solubility complications and stabilize the tear film [
306]. Nanoemulsions were prepared by microfluidization, using lecithin as an anionic surfactant, Miglyol 812 as an internal oil phase, Kolliphor EL as the second surfactant, and CS. CS was expected to depose on the surface of the nanoemulsion droplets, increasing the ζ-potential and thus mucoadhesion. The mucoadhesive properties of the obtained formulations were tested rheologically after mixing with mucin dispersion, and indeed, a significantly increased mucoadhesion was observed for the CS-coated nanoemulsion. The formulation further demonstrated good compatibility and stability. Nanoemulsions with 0.05%
w/w CS exhibited the best characteristics and were found to be adequate for ophthalmic applications.
Another group that worked on liposomes coated with CS is Zhang et al. [
307]. The group prepared liposomes via the reverse-phase evaporation method, which were subsequently coated with TMCS,
1 in
Scheme 1. Cyanidin-3-glucoside was encapsulated in the liposomes for the treatment of cataract. The uncoated liposomes were negatively charged while a modified CS coating made their surface positively charged and simulteneously enlarged their size. Furthermore, the coating protected the drug from oxidative damage. The presence of TMCS increased the pre-corneal retention time, which was triplicated compared to uncoated liposomes. Moreover, it enhanced the permeation and the transepithelial transport of liposomes, as it opened the tight junctions between the epithelial cells. Regarding the release of the drug, a slower release and diffusion of the drug was observed owing to the presence of the coating. Huang et al. also used TMCS to coat lanosterol and hesperetin-loaded liposomes for cataract prevention and treatment [
308]. The coated liposomes exhibited a slightly decreased encapsulation efficiency compared to the uncoated one. Most importantly, in in vivo studies, they demonstrated a smaller burst release and a slower overall release, extending over one week. Despite their lower drug load, the coated liposomes had a better therapeutic efficacy with no progression of cataract observed for the subjects treated with TMCS-coated liposomes.
Pai et al. used CS oligosaccharide in order to coat NLCs for the delivery of etoposide, which is an antineoplastic agent used for suppressing tumors that occur in the eye such as retinoblastoma [
309]. NLCs were prepared via a hot homogenization–ultrasonication technique, and the resulting NPs were coated by CS oligosaccharides. Typical findings were also verified in the present study, i.e., an increase of NP size after CS coating (from 103 to 117 nm) and the conversion of negatively charged NPs to positively charged ones. However, compared to other analogous formulations, it was found that the release profile of the drug, conducted in STF, was not affected by CS oligosaccharide coating, which was probably due to the form of CS used, i.e., oligosaccharides. The mucoadhesion properties of coated NPs were enhanced compared to the negligible mucoadhesion of non-coated NPs. Mucoadhesion prolonged the presence of NPs on the ocular surface, as revealed by in vivo studies, leading to an enhanced ocular concentration of etoposide in all parts of the eye.
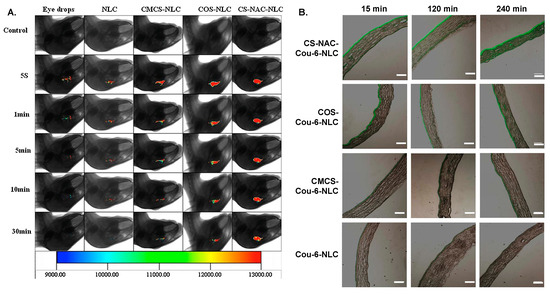
Li et al. studied three different CS derivatives—
N-acetylcysteine CS (NACCS),
6 in
Scheme 1, CS oligosaccharides, and carboxymethyl CS—for the coating of curcumin-loaded NLC [
310]. According to curcumin release studies, NACCS showed a remarkably slower drug release compared to the other two coatings, which was probably due to the formation of disulfide bonds within the coating, impeding the diffusion of the drug. CS oligosaccharides showed a faster release compared to the thiolated CS coating; however, in contrast to Pai et al. [
309], it was still slower than uncoated NLC. Finally, carboxymethyl CS showed the fastest release of all the coated NPs. Concerning corneal permeation studies, it was found that in the first 60 min, no difference was observed among the three coated formulations, but thereafter, thiolated CS, i.e., NACCS, showed an enhanced corneal permeation due to the thiol groups that can penetrate the tight junctions of mucus layers. These observations were further confirmed by fluorescence imaging, which was used to evaluate in vivo pre-corneal retention (A). The same team further investigated the influence of the thiol group content in NACCS, which was used as a coating for curcumin-loaded NLC [
311]. The total content of thiol groups did not affect curcumin’s release despite differences in the size and ζ-potential. This was attributed to the similar entrapment efficiency observed for all three coatings. Differences appeared in ex vivo cornea penetration studies, which showed that after 60 min, the coating with the highest degree of thiolation resulted in higher cornea penetration. These outcomes were also confirmed by fluorescence imaging. A similar study was conducted for the ocular delivery of hydrophobic coumarin-6 instead of curcumin [
312,
313]. The results concerning both the coating with NACCS compared to CS oligosaccharides or carboxymethyl CS [
312], as well as the content of thiol groups [
313], were the same (B).
Figure 22. (
A) In vivo fluorescence imaging of curcumin (CUR) in eye drops, nanostructured lipid carriers (NLC), carboxymethyl chitosan-coated NLC (CMCS-NLC), chitosan oligosaccharides-coated NLC (COS-NLC) and N-acetylcysteine chitosan-coated NLC (CS-NAC-NLC) at 5 s, 1 min, 5 min, 10 min, and 30 min after administration. Reprinted from ref. [
310]. Copyright 2016 with permission from Elsevier Ltd. (
B) Inverted fluorescence microscope micrographs after time-coursed in vivo corneal permeation of the aforementioned preparations, loaded with coumarin. Scale bar is 150 μm. Adapted from ref. [
312]. Copyright 2017, with permission from Elsevier B.V.
Salama et al. used PLGA 50/50 or 75/25 in NP formulation of fluocinolone acetonide (FA), which is an anti-inflammatory corticosteroid drug used for the treatment of intermediate, posterior, and pan uveitis [
314]. NPs were prepared by an innovative thin film hydration process using P407 in a mass ratio of PLGA/P407 1:5 and 1:10. The NPs prepared with PLGA 50/50 were smaller compared to the ones prepared with PLGA 75/25. This was attributed to the higher hydrophilicity of the former due to the higher glycolide content. This enabled the easier interfacial arrangement of PLGA molecules. Entrapment efficiency was higher for PLGA 75/25 NPs, which was due to the higher hydrophobic content of the polymer, which favored the encapsulation of the hydrophobic drug. The ζ-potential was negative for all prepared NPs due to the presence of terminal carboxylic acid groups in PLGA, and it increased with increasing poloxamer content. The authors selected the best formulation and proceeded in NPs coating using CS hydrochloride in different concentrations. It was observed, as it was by Seyfoddin et al. [
303], that as the concentration of CS increased, the size of NPs increased from about 203 nm to about 2147 nm, while the ζ-potential also increased from negative to positive values. The important increase in size was attributed to agglomeration. Unfortunately, the isolation procedure of NPs was not described; it was probably by freeze drying, which could explain such sizes. The release profile revealed that the coated NPs showed improvements compared to the uncoated ones. In contrast to FA, which is known to cause irritation, an ocular irritation study conducted in rabbit eyes demonstrated that neither uncoated nor coated NPs induced any irritation. Furthermore, it was found that the concentration of FA in rabbits tears were higher when coated NPs were used. This was mainly attributed to the mucoadhesive properties of CS.
Pandit et al. studied CS-coated PLGA NPs for bevacizumab delivery [
315]. Drug-loaded NPs were prepared by double emulsion solvent evaporation, experimental parameters were optimized by response surface methodology, and well-defined, spherical core–shell particles were obtained. CS coating contributed to a reduced initial burst release, a slower release compared to uncoated NPs, and a higher permeation. Khan et al. reported CS-coated PLGA NPs for the delivery of forskolin in glaucoma treatment [
316]. CS-coated NPs were prepared in a single step by emulsion sonication. The experimental parameters were optimized using the Box–Behnken design. Increasing the CS concentration resulted in an increase in NPs’ size, a rise in PDI, and a decrease in drug loading, but an increase in entrapment efficiency. The optimized NPs were approximately 200 nm and had a positive ζ-potential. A slow release of forskolin was observed with no burst release. According to the authors, CS coating functioned as a physical barrier, restricting drug release. It is suggested that drug release was a result of CS swelling/erosion, followed by PLGA erosion and drug diffusion. Due to CS coating, the NPs were found to penetrate deeper in the cornea. Overall, CS-coated forskolin-loaded PLGA NPs showed a sustained IOP decrease for 24 h. Dyawanapelly et al. investigated the potential of CS oligosaccharide in the mucosal delivery of drug-loaded PLGA NPs [
317]. In contrast to CS coating, CS oligosaccharide coating did not affect significantly drug release. Both coatings resulted in improved mucoadhesion and higher cellular uptake. Mahaling et al. studied the influence of different coatings on the bioavailability and distribution of NPs in the ocular region [
318,
319]. Initially, CS, gelatin, or pluronic F68 coatings of poly(ε-caprolactone) (PCL) NPs were studied, and the scope was then further extended to PLA and PLGA NPs. The NPs coated with F68 exhibited the highest hydrophilicity and mucoadhesivity. For PCL NPs, F68 was the best coating, but for PLGA NPs, CS-coated NPs demonstrated a higher bioavailability in conjunctiva, sclera, choroid, and retina. Nasr and Khoee reported the formation of a crosslinked CS shell, coating poly(butylene adipate) (PBA) micelles for the improved ocular delivery of loteprednol etabonate [
320]. PBA was modified in order to prepare a dendrimerized structure with hydrophilic amine-functionalized chain ends that could form micelles. The amine-functionalized micelles were further reacted with
N-succinyl modified CS,
2 in
Scheme 1, in the presence of EDC and
N-hydroxysuccinimide. CS was grafted to the micelles via the reaction of the amine groups of the micelles with the carboxylic acid groups of modified CS. Additionally, a crosslinking reaction among amine and carboxylic groups within CS chains took also place. Core–shell spherical NPs were obtained with sizes from 40 to 82 nm depending on the molecular weight of PBA. Commercial eye drops released their drug content within 10 h. In contrast, loteprednol etabonate release from the crosslinked core–shell NPs was extended over 50 h.