Harnessing solar energy and converting it into renewable fuels by chemical processes, such as water splitting and carbon dioxide (CO2) reduction, is a highly promising yet challenging strategy to mitigate the effects arising from the global energy crisis and serious environmental concerns. In recent years, covalent organic framework (COF)-based materials have gained substantial research interest because of their diversified architecture, tunable composition, large surface area, and high thermal and chemical stability. Their tunable band structure and significant light absorption with higher charge separation efficiency of photoinduced carriers make them suitable candidates for photocatalytic applications in hydrogen (H2) generation, CO2 conversion, and various organic transformation reactions.
- covalent organic frameworks
- photocatalysis
- CO2 reduction and water splitting
1. Introduction
2. COFs-Based Hybrids for Photocatalytic H2 Generation
| COFs | Synthesis | Novelty | Ref. |
|---|---|---|---|
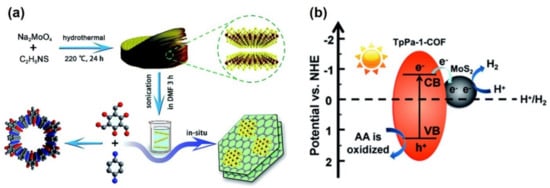
| MoS2/TpPa-1-COF | In situ growth of COF in an exfoliated MoS2 dispersion in DMF | MoS2 nanosheets evenly distributed on flower-like cluster TpPa-1-COF | [53] |
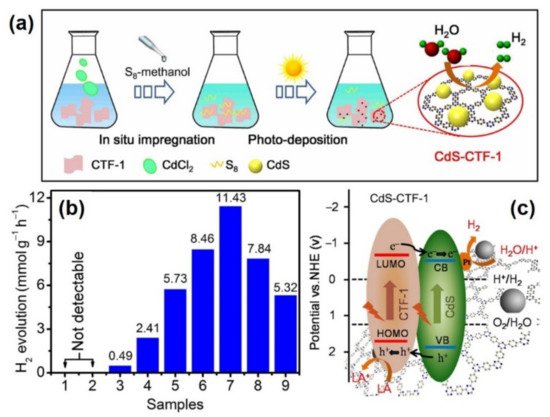
| CdS-CTF-1 | Impregnation combined with photodeposition approach to disperse CdS NPs on triazine based COF | Smaller sized CdS NPs with more exposed active sites | [54] |
| TTR-COF | Condensation of 1,3,5-tris(4-formylphenyl)triazine (TFPT) and 2,5-bis(2-ethylthio)ethoxy)terephthalohydrazide (BETH) under solvothermal conditions | Selective adsorption of Au through affinity of thioether functionalized COF | [55] |
| (thioether functionalized) | |||
| g-C18N3-COF and g-C33N3-COF | Knoevenagel condensation approach with linear and trigonal aldehyde monomers to form g-C18N3-COF and g-C33N3-COF, respectively | Honeycomb-like porous COF structures with sp2 carbon-linked triazine units | [56] |
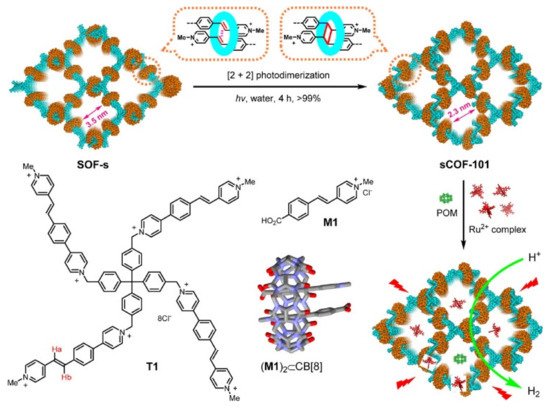
| sCOF-101 | An irreversible polymerization of supramolecular organic framework through cucurbit [8] uril [2+2] photodimerization | 3D ordered porous COF with a channel diameter of 2.3 nm, capable of enriching photosensitizer and redox-active sites in water | [57] |
| N2-COF | Solvothermal acid catalyzed condensation between 1,3,5-triformylbenzene (TFB) and 2,5-diethoxyterephthalohydrazide (DETH) | An outer sphere of electron transfer from azine-linked N2-COF to cobaloxime co-catalyst, as revealed by quantum calculations | [58] |
| Cobaloxime immobilized COF-42 | DETH was replaced with propargyl-containing 2,5-bis(prop-2-yn-1-yloxy)terephthalohydrazide (DPTH) to provide functional sites for covalent attachment of cobaloxime by click-chemistry approach | Propargyl-functionalized COF-42 with covalently tethered three different cobaloxime to form COF−cobaloxime hybrid catalyst | [59] |
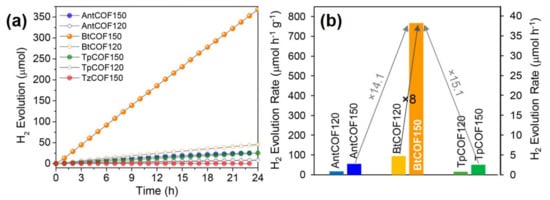
| BtCOF-150 | Substitution of the central benzene ring of TpCOF with benzothiazole (Bt) at 150 °C | The AA’ stacked BtCOF150 displayed improved charge migration | [60] |
| ATNT hybrid | Mixture of COF and NH2-Ti3C2Tx ultrasonicated followed by heating at 120° C for 3 days to form a dark red precipitate | The COF forms oriented 2D layers parallel to the substrate surface with vertically aligned π-columns in the ATNT hybrid | [61] |
| COFs | Type | Light Source | Reaction Conditions | Photocatalytic Performance (µmolh−1g−1) |
Ref. |
|---|---|---|---|---|---|
| ATNT hybrid | Ketoenamine-based | 300 W Xe lamp (λ > 420 nm) |
Catalyst (10 mg), ascorbic acid (100 mg), 3 wt % Pt as cocatalyst | 14,228 | [61] |
| CdS-CTF-1 | Covalent triazine-based | 300 W Xe (λ > 420 nm) |
Catalyst (20 mg), lactic acid (8 mL), water (80 mL) | 11,430 | [54] |
| MoS2/TpPa-1-COF | Ketoenamine-based | 300 W Xe (λ > 420 nm) |
Catalyst (10 mg), ascorbic acid (100 mg), water (50 mL) | 5585 | [53] |
| N2-COF | Hydrazone-based | 300 W Xe lamp (I = 100 mW cm−2) |
Catalyst (5 mg), triethanolamine (100 µL), acetonitrile (10 mL), chloro(pyridine)cobaloxime (400 µL, 2.48 mM) as co-catalyst | 782 | [58] |
| BtCOF-150 (Bt: benzothiadiazole) | Ketoenamine-based | 300 W Xe lamp (λ > 400 nm) |
Catalyst (20 mg), 1 wt % Pt, water−TEOA (4:1, 100 mL, pH 11) | 750 | [60] |
| g-C18N3-COF | 2D triazine-based | 300 W Xe (λ > 420 nm) |
Catalyst (50 mg), ascorbic acid (100 mL, 1 M), 3 wt % Pt as cocatalyst | 292 | [56] |
| Cobaloxime immobilized COF-42 | Hydrazone-based | 300 W Xe lamp (I = 100 mW cm−2) |
Catalyst (5 mg), acetonitrile (10 mL), triethanolamine (100 µL) | 163 | [59] |
| TTR-COF (thioether functionalized) |
Covalent triazine-based | 300 W Xe (λ > 420 nm) |
Catalyst (20 mg), seawater (50 mL), triethanolamine (5 mL) | 141 | [55] |
| sCOF-101 (water soluble) |
3D-supramolecular framework-based | 300 W solid state light | Methanol (2 mL), pH = 1.8, [Ru(bpy)3]2+, POM | TON = 400 | [57] |
| COFs | Active Sites | Topology | Photocatalytic Performance (µmolh−1g−1) |
Ref. |
|---|---|---|---|---|
| MoS2/TpPa-1-COF | TpPa-1-COF: light absorber | Nanoflower-like morphology of MoS2 sheets | 5585 | [53] |
| MoS2: Reductant | ||||
| CdS-CTF-1 | CTF-1, CdS: light absorber | Small sized CdS NPs leads to more active sites and superior activity | 11,430 | [54] |
| Pt: cocatalyst, reductant | ||||
| TTR-COF | TTR COF: light absorber | Selective adsorption of Au through affinity of thioether functionalized COF | 141 | [55] |
| (thioether functionalized) | Plasmonic Au NPs: reductant | |||
| g-C18N3-COF and g-C33N3-COF | Olefin linked COF: photosensitizer and active site | Honeycomb-like porous COF structures with sp2 carbon-linked triazine units | 292 | [56] |
| sCOF-101 | COF: light absorber | 3D ordered porous COF with a channel diameter of 2.3 nm, capable of enriching photosensitizer and redox-active sites in water | TON = 400 | [57] |
| Ru2+ complex: photosensitizer | ||||
| Polyoxometalate: redox active sites | ||||
| N2-COF | COF: photosensitizer | An outer sphere of electron transfer from azine-linked N2-COF to cobaloxime co-catalyst, as revealed by quantum calculations | 782 | [58] |
| Cobaloxime: co-catalyst (redox-active) | ||||
| Cobaloxime immobilized COF-42 | COF: photosensitizer | Propargyl-functionalized COF-42 with covalently tethered three different cobaloxime to form COF−cobaloxime hybrid catalyst | 163 | [59] |
| Cobaloxime: active site | ||||
| BtCOF-150 | COF: photosensitizer | The AA’ stacked layers of BtCOF150 displayed improved charge migration | 750 | [60] |
| Pt: cocatalyst (reductant) | ||||
| ATNT hybrid | COF: photosensitizer | The 2D parallel layers of COF to the surface with vertically aligned π columns in the ATNT hybrid | 14,228 | [61] |
| MXene: electron transfer mediator | ||||
| Pt: cocatalyst, reductant |
| Photocatalyst | Light Source | Reaction Conditions | Photocatalytic Performance (µmolg−1h−1) |
Ref. |
|---|---|---|---|---|
| Au-Ag/TiO2 | 250 W Hg lamp | Catalyst (14 mg), 20 vol % methanol in water | 12,820 | [63] |
| Ni-Cd/CdS | 300 W Xe lamp (λ > 410 nm) | Catalyst (20 mg), Na2S (0.1 M), Na2SO3 (0.1 M) | 11,570 | [64] |
| FeCu(1:1)@C/g-C3N4 | 300 W Xe lamp | Catalyst (10 mg), 15 % TEOA in 100 mL water | 722 | [65] |
| Ni2P/ZnIn2S4 | 300 W Xe lamp (λ > 400 nm | Catalyst (50 mg), 10 vol % lactic acid in 100 mL water | 2066 | [66] |
| Cu-nanodiamond | 300 W Xe lamp | Catalyst (100 mg), 20 vol % ethanol | 1597 | [67] |
| C3N4-MoS2 nanocomposite | 400 W Xe lamp | Catalyst (3 mg), 20 vol % TEOA in 40 mL water | 12,778 | [68] |
3. COFs-Based Hybrids for Photocatalytic CO2 Conversion
| COFs | Synthesis | Novelty | Ref. |
|---|---|---|---|
| Co-CTF-1 | Aerobic acid catalyzed trimerization of terephthalonitrile, with wetness impregnation of Co | 2D-layered COF porous structure with triazine units promoting CO2 adsorption and accommodation | [69] |
| [Ni(bipy)3]2+—PI-COF | Anaerobic solvothermal imide condensation between pyromellitic dianhydride and amine-terminated building units. | Hexagonal porous polyimide COF assisting in CO2 photoreduction | [70] |
| N3-COF, ACOF-1 | Anaerobic solvothermal condensation between triformylbenzene and hydrazine hydrate (ACOF-1), and 2,4,6-tris(4-bromophenyl)-1,3,5-triazine with N-formylpiperidine (N3COF) | Coplanar azine and phenyl rings facilitating the conjugation effect | [71] |
| Re-bpy-sp2c-COF | Knoevenagel condensation of 1,3,6,8-tetrakis(4-formylphenyl)pyrene and 5,5′-bis(cyanomethyl)-2,2′-bipyridine. Post synthetic ligation of [Re(CO)5Cl]. | Porous crystalline COF with bipyridine units ligated to Re complex, forming fully π-conjugated backbone | [11] |
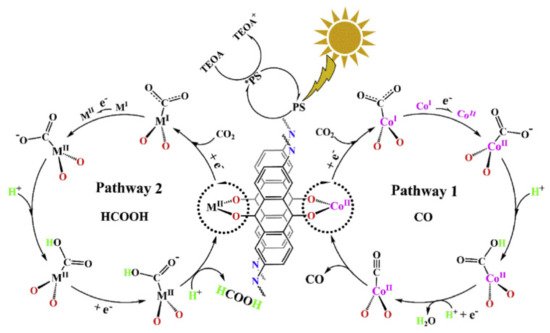
| COF-367-CoII/III | Low-pressure solvothermal (50 mTorr). Schiff base condensation between CoII/III-TAPP (5,10,15,20-Tetrakis(4-aminophenyl)porphyrin and 4, 4′-biphenyldicarboxaldehyde. | CoII and CoIII centered in porphyrin-based COF | [72] |
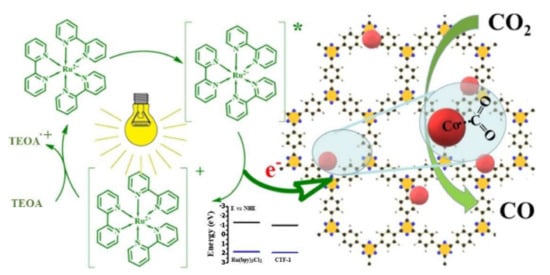
| Ru-TpPa-1 | Anaerobic solvothermal Schiff base condensation between 1,3,5-triformylphloro-glucinol and p-phenylenediamine. RuCl3 added post-synthetically through incipient wetness, followed by reduction in H2/N2. | Ketoamine-based COF loaded with Ru NPs enhancing the light absorption and charge separation | [73] |
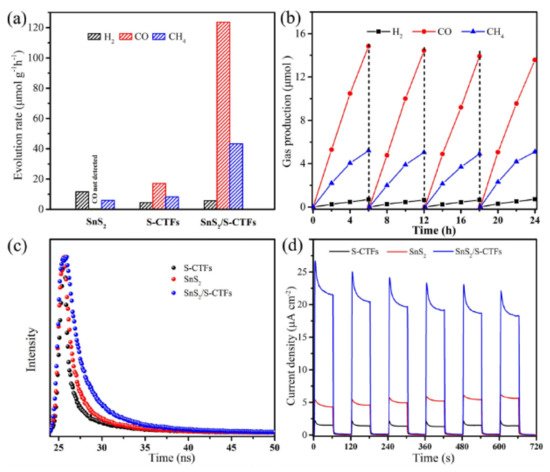
| Z-Scheme SnS2/S-CTF | Anaerobic solvothermal nucleophilic substitution between trithiocyanuric acid and cyanuric chloride. SnS2 nanoparticles grown on framework through addition of SnCl2 and heating under autogenous pressure with EtOH. | Flower-like surface made of vertically aligned nanosheets in which SnS2 nanocrystals are attached with S-CTF framework | [74] |
| Re-TpBpy | Anaerobic solvothermal Schiff base condensation between 1,3,5-tri- formylphloroglucinol and 2,2′-bipyridine-5,5′-diamine. Post synthetic ligation of [Re(CO)5Cl]. | Bipyridine units in COFs facilitating chelation with Re complex creating active sites on the porous walls | [75] |
| COF-367-Co Nanosheets | Solvothermal modulated imine exchange, between (5,10,15,20-Tetrakis(4-aminophenyl)porphyrin, 2,4,6-trimethylbenzaldehyde, and 4,4′-biphenyldialdehyde. | Ultrathin 2D imine-based Co-COF nanosheet structure with large surface area | [76] |
| DQTP- TMI | Solvothermal Schiff base condensation between 2,6-diaminoanthraquinone and 1,3,5-triformylphloroglucinol. Transition metal ions (TMIs) Co, Ni and Zn added post-synthetically via hydrothermal treatment. | 2D anthraquinone based COF with conjugation and porosity assisting electron movement and CO2 adsorption | [77] |
| COFs | Type | Light Source | Reaction Conditions | Photocatalytic Performance (μmol h−1 g−1) |
Ref. |
|---|---|---|---|---|---|
| COF-367-Co Nanosheets |
Bipyridyl-imine-linked porphyrinic | 300 W Xe (λ > 420 nm) |
Catalyst (5 mg) 20 mL KOH (aq) (0.1 M) 19 mg [Ru(bpy)3]Cl2.6H2O 352 mg ascorbic acid 1 atm CO2, 25 °C |
Nanosheet: 10,162 (CO) 2875 (H2) Bulk: 124 (CO) |
[76] |
| Re-bpy-sp2c-COF | 2D sp2c bipyridyl | 300 W Xe (λ > 420 nm) |
Catalyst (1 mg) 5 mL, 30:1 MeCN:TEOA 1 atm CO2 |
1040 (CO) | [11] |
| DQTP- TMI | 2D anthraquinone | 300 W Xe (λ > 420 nm) |
Catalyst (20 mg) 50 mL, 4:1 MeCN:TEOA 22.5 mg Ru(bpy)3Cl2.H2O 1 atm CO2, 25 °C |
TMI = Co: 1022 (CO) | [77] |
| [Ni(bipy)3]2+—PI-COF | Covalent Triazine | 300 W Xe (λ > 420 nm) |
Catalyst (10 mg) Ni(ClO4)2.6H2O (2 mg) 2,2′-bipyridyl (15 mg) 5 mL, 3:1:1 MeCN: H2O:TEOA 1 atm CO2 |
483 (CO) | [70] |
| Re-TpBpy | 2D bipyridyl-linked | 200 W Xe (λ > 390 nm) |
Catalyst (15 mg) 11.8 mL MeCN/H2O (10/1.8) 0.1 M TEOA 1 atm CO2, 25 °C Purpose-built reactor |
291.7 (CO) | [75] |
| Z-Scheme SnS2/S-CTF | Covalent Triazine | 300 W Xe (λ > 420 nm) |
Catalyst (20 mg), 10 mL, 4:1 H2O:TEOA 1 atm CO2, 25 °C |
123.6 (CO) 43.4 (CH4) |
[74] |
| Ru-TpPa-1 | Ketoamine-based | 300 W Xe (λ > 420 nm) |
Catalyst (15 mg) 110 mL, 10:1 MeCN:TEOA 1 atm CO2, |
0 wt % Ru: 32.4 (HCOOH) 1 wt % Ru: 41.8 (HCOOH) 3 wt % Ru: 108.8 (HCOOH) 5 wt % Ru: 61.8 (HCOOH) |
[73] |
| COF-367-CoII/III | Bipyridyl-imine-linked Porphyrinic | 300 W Xe (λ > 380 nm) |
Catalyst (10 mg) 22 mL, 10:1 MeCN:TEA 1 atm CO2 |
Co(II) 48.6 (HCOOH), 16.5 (CO), 12.8 (CH4) Co(III) 93.0 (HCOOH), 0.44 (CO), 10.1 (CH4) |
[72] |
| Co-CTF-T1 | Covalent Triazine | 300 W Xe (λ > 420 nm) |
Catalyst (10 mg) 7 mg [Ru(bpy)3]2+ 0.9 mL, 1:2:3 TEOA:H2O:MeCN 1 atm CO2 |
48 (CO) | [69] |
| N3-COF, ACOF-1 | Covalent Triazine and Azine inked | 500 W Xe (λ > 420 nm) |
Catalyst (10 mg) 5 mL H2O 4 atm CO2, 80 °C |
N3-COF: 0.57 (MeOH) ACOF-1: 0.36 (MeOH) |
[71] |
| COFs | Active Sites | Topology | Photocatalytic Performance (µmolh−1g−1) | Ref. |
|---|---|---|---|---|
| Co-CTF-1 | COF and Ru(bpy)32+: photosensitizer Co: redox active sites |
2D-layered COF porous structure with triazine units promoting CO2 adsorption and accommodation | 48 (CO) | [69] |
| [Ni(bipy)3]2+—PI-COF | COF: photosensitizer Ni: CO2 activation |
Hexagonal porous polyimide COF assisting in CO2 photoreduction | 483 (CO) | [70] |
| N3-COF, ACOF-1 | COF: photosensitizer and redox active site | Coplanar azine and phenyl rings facilitating the conjugation effect | N3-COF: 0.57 (MeOH) ACOF-1: 0.36 (MeOH) |
[71] |
| Re-bpy-sp2c-COF | COF: photosensitizer Re complex: electron mediator |
Porous crystalline COF with bipyridine units ligated to Re complex, forming fully π-conjugated backbone | 1040 (CO) | [11] |
| COF-367-CoII/III | COF: photosensitizer Co: redox active site |
CoII and CoIII centered in porphyrin-based COF | Co(II) 48.6 (HCOOH), 16.5 (CO), 12.8 (CH4) Co(III) 93.0 (HCOOH), 0.44 (CO), 10.1 (CH4) |
[72] |
| Ru-TpPa-1 | COF: photosensitizer Ru NPs: reductant |
Ketoamine-based COF loaded with Ru NPs enhancing the light absorption and charge separation | 0 wt % Ru: 32.4 (HCOOH) 1 wt % Ru: 41.8 (HCOOH) 3 wt % Ru: 108.8 (HCOOH) 5 wt % Ru: 61.8 (HCOOH) |
[73] |
| Z-Scheme SnS2/S-CTF | SnS2 and COF: photosensitizer COF: CO2 adsorption and activation |
Flower-like surface made of vertically aligned nanosheets in which SnS2 nanocrystals are attached with S-CTF framework | 123.6 (CO) 43.4 (CH4) |
[74] |
| Re-TpBpy | COF: photosensitizer Re complex: catalytic active site |
Bipyridine units in COFs facilitating chelation with Re complex creating active sites on the porous walls | 291.7 (CO) | [75] |
| COF-367-Co Nanosheets | Ru complex: photosensitizer Co-COF: active site |
Ultrathin 2D imine-based Co-COF nanosheet structure with large surface area | Nanosheet: 10162 (CO) 2875 (H2) Bulk: 124 (CO) |
[76] |
| DQTP- TMI | Ru complex: photosensitizer Co, Ni, Zn-COF: redox active site for CO2 adsorption and reduction |
2D anthraquinone based COF with conjugation and porosity assisting electron movement and CO2 adsorption | TMI = Co: 1022 (CO) | [77] |
| Photocatalyst | Light Source | Reaction Conditions | Photocatalytic Performance (µmolh−1g−1) | Ref |
|---|---|---|---|---|
| Ag-Cr/Ga2O3 | 400 W Hg lamp (λ > 420 nm) | Catalyst (0.5 g), NaHCO3 (0.1 M), CO2 (30 mLmin−1) | 480 (CO) | [85] |
| Cu@V-TiO2/PU | Two white light bulbs | CO2 (50 mLmin−1) passing through water at 303 K | 933 (CH4) | [86] |
| ZnO nanosheets | 125 W Hg lamp | Catalyst (50 mg), 0.5 mL deionized water at 473 K, CO2 (1 atm) | 406.77 (CO) 20.16 (CH4) |
[87] |
| Rh-Au-SrTiO3 | 500 W Xe lamp (λ > 400 nm) |
Catalyst (75 mg), 3 mL deionized water and CO2 (70 kPa) | 369 (CO) | [88] |
| Mo-doped g-C3N4 | 300 W Hg lamp | Catalyst (100 mg), 5.0 g deionized water at 303 K and CO2 (110 KPa) | 887 (CO) 123 (CH4) |
[89] |
| Nb-TiO2/g-C3N4 | Two 30 W white bulbs | Catalyst (100 mg), CO2 (20 mL min−1) passing through water at 303 K | 562 (CH4) 420 (CO) 698 (HCOOH) |
[90] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules26144181
References
- Keller, N.; Bessinger, D.; Reuter, S.; Calik, M.; Ascherl, L.; Hanusch, F.C.; Auras, F.; Bein, T. Oligothiophene-Bridged Conjugated Covalent Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 8194–8199.
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 1–19.
- Liu, R.; Tan, K.T.; Gong, Y.; Chen, Y.; Li, Z.; Xie, S.; He, T.; Lu, Z.; Yang, H.; Jiang, D. Covalent organic frameworks: An ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 2021, 50, 120–242.
- Ockwig, N.W.; Co, A.P.; Keeffe, M.O.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1171.
- Guo, L.; Jin, S. Stable Covalent Organic Frameworks for Photochemical Applications. ChemPhotoChem 2019, 3, 973–983.
- Sharma, R.K.; Yadav, P.; Yadav, M.; Gupta, R.; Rana, P.; Srivastava, A.; Zbořil, R.; Varma, R.S.; Antonietti, M.; Gawande, M.B. Recent development of covalent organic frameworks (COFs): Synthesis and catalytic (organic-electro-photo) applications. Mater. Horiz. 2020, 7, 411–454.
- Cheng, H.Y.; Wang, T. Covalent Organic Frameworks in Catalytic Organic Synthesis. Adv. Synth. Catal. 2021, 363, 144–193.
- You, J.; Zhao, Y.; Wang, L.; Bao, W. Recent developments in the photocatalytic applications of covalent organic frameworks: A review. J. Clean. Prod. 2021, 291, 125822.
- Wang, H.; Wang, H.; Wang, Z.; Tang, L.; Zeng, G.; Xu, P.; Chen, M.; Xiong, T.; Zhou, C.; Li, X.; et al. Covalent organic framework photocatalysts: Structures and applications. Chem. Soc. Rev. 2020, 49, 4135–4165.
- Bagheri, A.R.; Aramesh, N. Towards the room-temperature synthesis of covalent organic frameworks: A mini-review. J. Mater. Sci. 2021, 56, 1116–1132.
- Fu, Z.; Wang, X.; Gardner, A.M.; Wang, X.; Chong, S.Y.; Neri, G.; Cowan, A.J.; Liu, L.; Li, X.; Vogel, A.; et al. A stable covalent organic framework for photocatalytic carbon dioxide reduction. Chem. Sci. 2020, 11, 543–550.
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933.
- Yang, Q.; Luo, M.; Liu, K.; Cao, H.; Yan, H. Covalent organic frameworks for photocatalytic applications. Appl. Catal. B Environ. 2020, 276, 119174.
- Sajjad, M.; Lu, W. Covalent organic frameworks based nanomaterials: Design, synthesis, and current status for supercapacitor applications: A review. J. Energy Storage 2021, 39, 102618.
- Huang, J.; Liu, X.; Zhang, W.; Liu, Z.; Zhong, H.; Shao, B.; Liang, Q.; Liu, Y.; He, Q. Functionalization of covalent organic frameworks by metal modification: Construction, properties and applications. Chem. Eng. J. 2021, 404, 127136.
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868.
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022.
- Abuzeid, H.R.; EL-Mahdy, A.F.M.; Kuo, S.-W. Covalent organic frameworks: Design principles, synthetic strategies, and diverse applications. Giant 2021, 6, 100054.
- Zhi, Y.; Wang, Z.; Zhang, H.L.; Zhang, Q. Recent Progress in Metal-Free Covalent Organic Frameworks as Heterogeneous Catalysts. Small 2020, 16, 1–21.
- Sinha, N.; Pakhira, S. Tunability of the Electronic Properties of Covalent Organic Frameworks. ACS Appl. Electron. Mater. 2021, 3, 720–732.
- Fang, Q.; Gu, S.; Zheng, J.; Zhuang, Z.; Qiu, S.; Yan, Y. 3D Microporous Base-Functionalized Covalent Organic Frameworks for Size-Selective Catalysis. Angew. Chem. 2014, 126, 2922–2926.
- Fan, M.; Wang, W.D.; Zhu, Y.; Sun, X.; Zhang, F.; Dong, Z. Palladium clusters confined in triazinyl-functionalized COFs with enhanced catalytic activity. Appl. Catal. B Environ. 2019, 257, 117942.
- Wang, J.; Zhuang, S. Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev. 2019, 400, 213046.
- Li, Z.; Feng, X.; Zou, Y.; Zhang, Y.; Xia, H.; Liu, X.; Mu, Y. A 2D azine-linked covalent organic framework for gas storage applications. Chem. Commun. 2014, 50, 13825–13828.
- Li, Z.; Zhi, Y.; Feng, X.; Ding, X.; Zou, Y.; Liu, X.; Mu, Y. An Azine-Linked Covalent Organic Framework: Synthesis, Characterization and Efficient Gas Storage. Chem. Eur. J. 2015, 21, 12079–12084.
- Xie, Z.; Wang, B.; Yang, Z.; Yang, X.; Yu, X.; Xing, G.; Zhang, Y.; Chen, L. Stable 2D Heteroporous Covalent Organic Frameworks for Efficient Ionic Conduction. Angew. Chem. 2019, 131, 15889–15893.
- Li, X.; Loh, K.P. Recent Progress in Covalent Organic Frameworks as Solid-State Ion Conductors. ACS Mater. Lett. 2019, 1, 327–335.
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363.
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Design of silver-based controlled nanostructures for plasmonic catalysis under visible light irradiation. Bull. Chem. Soc. Jpn. 2019, 92, 19–29.
- Verma, P.; Stewart, D.J.; Raja, R. Recent Advances in Photocatalytic CO2 Utilisation Over Multifunctional Metal—Organic Frameworks. Catalysts 2020, 10, 1176.
- Newland, S.H.; Sinkler, W.; Mezza, T.; Bare, S.R.; Carravetta, M.; Haies, I.M.; Levy, A.; Keenan, S.; Raja, R. Expanding beyond the Micropore: Active-Site Engineering in Hierarchical Architectures for Beckmann Rearrangement. ACS Catal. 2015, 5, 6587–6593.
- Chapman, S.; Carravetta, M.; Miletto, I.; Doherty, C.M.; Dixon, H.; Taylor, J.D.; Gianotti, E.; Yu, J.; Raja, R. Probing the Design Rationale of a High-Performing Faujasitic Zeotype Engineered to have Hierarchical Porosity and Moderated Acidity. Angew. Chem. Int. Ed. 2020, 59, 19561–19569.
- Verma, P.; Potter, M.E.; Oakley, A.E.; Mhembere, P.M.; Raja, R. Bimetallic PdAu Catalysts within Hierarchically Porous Architectures for Aerobic Oxidation of Benzyl Alcohol. Nanomaterials 2021, 11, 350.
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329.
- Navlani-García, M.; Verma, P.; Kuwahara, Y.; Kamegawa, T.; Mori, K.; Yamashita, H. Visible-light-enhanced catalytic activity of Ru nanoparticles over carbon modified g-C3N4. J. Photochem. Photobiol. A Chem. 2018, 358, 327–333.
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A Belt-Shaped, Blue Luminescent, and Semiconducting Covalent Organic Framework. Angew. Chem. 2008, 120, 8958–8962.
- Kuecken, S.; Acharjya, A.; Zhi, L.; Schwarze, M.; Schomäcker, R.; Thomas, A. Fast tuning of covalent triazine frameworks for photocatalytic hydrogen evolution. Chem. Commun. 2017, 53, 5854–5857.
- Li, L.; Fang, W.; Zhang, P.; Bi, J.; He, Y.; Wang, J.; Su, W. Sulfur-doped covalent triazine-based frameworks for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. A 2016, 4, 12402–12406.
- Cheng, Z.; Fang, W.; Zhao, T.; Fang, S.; Bi, J.; Liang, S.; Li, L.; Yu, Y.; Wu, L. Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution on Phosphorus-Doped Covalent Triazine-Based Frameworks. ACS Appl. Mater. Interfaces 2018, 10, 41415–41421.
- Meier, C.B.; Sprick, R.S.; Monti, A.; Guiglion, P.; Lee, J.S.M.; Zwijnenburg, M.A.; Cooper, A.I. Structure-property relationships for covalent triazine-based frameworks: The effect of spacer length on photocatalytic hydrogen evolution from water. Polymer 2017, 126, 283–290.
- Liu, S.; Tian, M.; Bu, X.; Tian, H.; Yang, X. Covalent Organic Frameworks toward Diverse Photocatalytic Aerobic Oxidations. Chem. Eur. J. 2021, 27, 7738–7744.
- Almansaf, Z.; Hu, J.; Zanca, F.; Shahsavari, H.R.; Kampmeyer, B.; Tsuji, M.; Maity, K.; Lomonte, V.; Ha, Y.; Mastrorilli, P.; et al. Pt(II)-Decorated Covalent Organic Framework for Photocatalytic Difluoroalkylation and Oxidative Cyclization Reactions. ACS Appl. Mater. Interfaces 2021, 13, 6349–6358.
- Lv, S.W.; Liu, J.M.; Yang, F.E.; Li, C.Y.; Wang, S. A novel photocatalytic platform based on the newly-constructed ternary composites with a double p-n heterojunction for contaminants degradation and bacteria inactivation. Chem. Eng. J. 2021, 409, 128269.
- Elmetwally, A.E.; Zeynaloo, E.; Shukla, D.; Surnar, B.; Dhar, S.; Cohn, J.L.; Knecht, M.R.; Bachas, L.G. Cu2O Cubes Decorated with Azine-Based Covalent Organic Framework Spheres and Pd Nanoparticles as Tandem Photocatalyst for Light-Driven Degradation of Chlorinated Biphenyls. ACS Appl. Nano Mater. 2021, 4, 2795–2805.
- Lv, H.; Zhao, X.; Niu, H.; He, S.; Tang, Z.; Wu, F.; Giesy, J.P. Ball milling synthesis of covalent organic framework as a highly active photocatalyst for degradation of organic contaminants. J. Hazard. Mater. 2019, 369, 494–502.
- Chen, H.; Liu, W.; Laemont, A.; Krishnaraj, C.; Feng, X.; Rohman, F.; Meledina, M.; Zhang, Q.; Van Deun, R.; Leus, K.; et al. A Visible-Light-Harvesting Covalent Organic Framework Bearing Single Nickel Sites as a Highly Efficient Sulfur–Carbon Cross-Coupling Dual Catalyst. Angew. Chem. Int. Ed. 2021, 60, 10820–10827.
- Verma, P.; Mori, K.; Kuwahara, Y.; Raja, R.; Yamashita, H. Plasmonic nanocatalysts for visible-NIR light induced hydrogen generation from storage materials. Mater. Adv. 2021, 2, 880–906.
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.A.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 2019, 4, 746–760.
- Vyas, V.S.; Haase, F.; Stegbauer, L.; Savasci, G.; Podjaski, F.; Ochsenfeld, C.; Lotsch, B.V. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 2015, 6, 1–9.
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B.V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793.
- Stegbauer, L.; Zech, S.; Savasci, G.; Banerjee, T.; Podjaski, F.; Schwinghammer, K.; Ochsenfeld, C.; Lotsch, B.V. Tailor-Made Photoconductive Pyrene-Based Covalent Organic Frameworks for Visible-Light Driven Hydrogen Generation. Adv. Energy Mater. 2018, 8, 1–8.
- Ding, S.Y.; Wang, P.L.; Yin, G.L.; Zhang, X.; Lu, G. Energy transfer in covalent organic frameworks for visible-light-induced hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 11872–11876.
- Gao, M.Y.; Li, C.C.; Tang, H.L.; Sun, X.J.; Dong, H.; Zhang, F.M. Boosting visible-light-driven hydrogen evolution of covalent organic frameworks through compositing with MoS2: A promising candidate for noble-metal-free photocatalysts. J. Mater. Chem. A 2019, 7, 20193–20200.
- Wang, D.; Zeng, H.; Xiong, X.; Wu, M.F.; Xia, M.; Xie, M.; Zou, J.P.; Luo, S.L. Highly efficient charge transfer in CdS-covalent organic framework nanocomposites for stable photocatalytic hydrogen evolution under visible light. Sci. Bull. 2020, 65, 113–122.
- Li, L.; Zhou, Z.; Li, L.; Zhuang, Z.; Bi, J.; Chen, J.; Yu, Y.; Yu, J. Thioether-Functionalized 2D Covalent Organic Framework Featuring Specific Affinity to Au for Photocatalytic Hydrogen Production from Seawater. ACS Sustain. Chem. Eng. 2019, 7, 18574–18581.
- Wei, S.; Zhang, W.; Qiang, P.; Yu, K.; Fu, X.; Wu, D.; Bi, S.; Zhang, F. Semiconducting 2D Triazine-Cored Covalent Organic Frameworks with Unsubstituted Olefin Linkages. J. Am. Chem. Soc. 2019, 141, 14272–14279.
- Gao, Z.Z.; Wang, Z.K.; Wei, L.; Yin, G.; Tian, J.; Liu, C.Z.; Wang, H.; Zhang, D.W.; Zhang, Y.B.; Li, X.; et al. Water-Soluble 3D Covalent Organic Framework that Displays an Enhanced Enrichment Effect of Photosensitizers and Catalysts for the Reduction of Protons to H2. ACS Appl. Mater. Interfaces 2020, 12, 1404–1411.
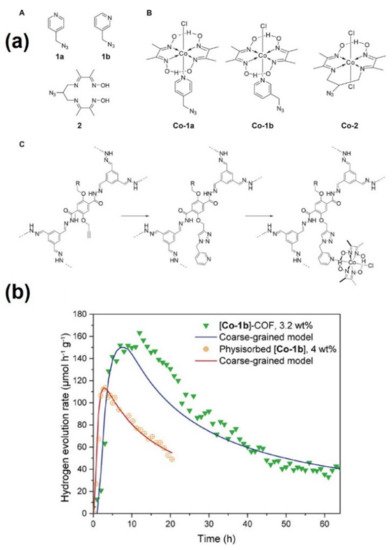
- Banerjee, T.; Haase, F.; Savasci, G.; Gottschling, K.; Ochsenfeld, C.; Lotsch, B.V. Single-Site Photocatalytic H2 Evolution from Covalent Organic Frameworks with Molecular Cobaloxime Co-Catalysts. J. Am. Chem. Soc. 2017, 139, 16228–16234.
- Gottschling, K.; Savasci, G.; Vignolo-González, H.; Schmidt, S.; Mauker, P.; Banerjee, T.; Rovó, P.; Ochsenfeld, C.; Lotsch, B.V. Rational Design of Covalent Cobaloxime-Covalent Organic Framework Hybrids for Enhanced Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2020, 142, 12146–12156.
- Ghosh, S.; Nakada, A.; Springer, M.A.; Kawaguchi, T.; Suzuki, K.; Kaji, H.; Baburin, I.; Kuc, A.; Heine, T.; Suzuki, H.; et al. Identification of Prime Factors to Maximize the Photocatalytic Hydrogen Evolution of Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 9752–9762.
- Wang, H.; Qian, C.; Liu, J.; Zeng, Y.; Wang, D.; Zhou, W.; Gu, L.; Wu, H.; Liu, G.; Zhao, Y. Integrating Suitable Linkage of Covalent Organic Frameworks into Covalently Bridged Inorganic/Organic Hybrids toward Efficient Photocatalysis. J. Am. Chem. Soc. 2020, 142, 4862–4871.
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481.
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190.
- Wang, B.; He, S.; Zhang, L.; Huang, X.; Gao, F.; Feng, W.; Liu, P. CdS nanorods decorated with inexpensive NiCd bimetallic nanoparticles as efficient photocatalysts for visible-light-driven photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 243, 229–235.
- Chen, S.; Li, M.; Yang, S.; Li, X.; Zhang, S. Graphitied carbon-coated bimetallic FeCu nanoparticles as original g-C3N4 cocatalysts for improving photocatalystic activity. Appl. Surf. Sci. 2019, 492, 571–578.
- Li, X.L.; Wang, X.J.; Zhu, J.Y.; Li, Y.P.; Zhao, J.; Li, F.T. Fabrication of two-dimensional Ni2P/ZnIn2S4 heterostructures for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2018, 353, 15–24.
- Lin, Z.; Xiao, J.; Li, L.; Liu, P.; Wang, C.; Yang, G. Nanodiamond-Embedded p-Type Copper(I) Oxide Nanocrystals for Broad-Spectrum Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1–10.
- Pramoda, K.; Gupta, U.; Chhetri, M.; Bandyopadhyay, A.; Pati, S.K.; Rao, C.N.R. Nanocomposites of C3N4 with Layers of MoS2 and Nitrogenated RGO, Obtained by Covalent Cross-Linking: Synthesis, Characterization, and HER Activity. ACS Appl. Mater. Interfaces 2017, 9, 10664–10672.
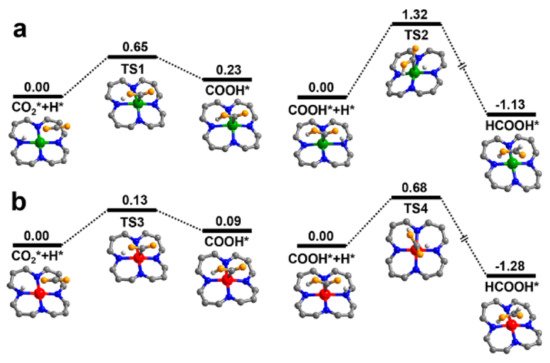
- Bi, J.; Xu, B.; Sun, L.; Huang, H.; Fang, S.; Li, L.; Wu, L. A Cobalt-Modified Covalent Triazine-Based Framework as an Efficient Cocatalyst for Visible-Light-Driven Photocatalytic CO2 Reduction. Chempluschem 2019, 84, 1149–1154.
- Chen, X.; Dang, Q.; Sa, R.; Li, L.; Li, L.; Bi, J.; Zhang, Z.; Long, J.; Yu, Y.; Zou, Z. Integrating single Ni sites into biomimetic networks of covalent organic frameworks for selective photoreduction of CO2. Chem. Sci. 2020, 11, 6915–6922.
- Fu, Y.; Zhu, X.; Huang, L.; Zhang, X.; Zhang, F.; Zhu, W. Azine-based covalent organic frameworks as metal-free visible light photocatalysts for CO2 reduction with H2O. Appl. Catal. B Environ. 2018, 239, 46–51.
- Gong, Y.N.; Zhong, W.; Li, Y.; Qiu, Y.; Zheng, L.; Jiang, J.; Jiang, H.L. Regulating photocatalysis by spin-state manipulation of cobalt in covalent organic frameworks. J. Am. Chem. Soc. 2020, 142, 16723–16731.
- Guo, K.; Zhu, X.; Peng, L.; Fu, Y.; Ma, R.; Lu, X.; Zhang, F.; Zhu, W.; Fan, M. Boosting photocatalytic CO2 reduction over a covalent organic framework decorated with ruthenium nanoparticles. Chem. Eng. J. 2021, 405, 127011.
- Guo, S.; Yang, P.; Zhao, Y.; Yu, X.; Wu, Y.; Zhang, H.; Yu, B.; Han, B.; George, M.W.; Liu, Z. Direct Z-Scheme Heterojunction of SnS2/Sulfur-Bridged Covalent Triazine Frameworks for Visible-Light-Driven CO2 Photoreduction. ChemSusChem 2020, 13, 6278–6283.
- Li, S.Y.; Meng, S.; Zou, X.; El-Roz, M.; Telegeev, I.; Thili, O.; Liu, T.X.; Zhu, G. Rhenium-functionalized covalent organic framework photocatalyst for efficient CO2 reduction under visible light. Microporous Mesoporous Mater. 2019, 285, 195–201.
- Liu, W.; Li, X.; Wang, C.; Pan, H.; Liu, W.; Wang, K.; Zeng, Q.; Wang, R.; Jiang, J. A Scalable General Synthetic Approach toward Ultrathin Imine-Linked Two-Dimensional Covalent Organic Framework Nanosheets for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 17431–17440.
- Lu, M.; Li, Q.; Liu, J.; Zhang, F.M.; Zhang, L.; Wang, J.L.; Kang, Z.H.; Lan, Y.Q. Installing earth-abundant metal active centers to covalent organic frameworks for efficient heterogeneous photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 254, 624–633.
- Eppinger, J.; Huang, K.W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195.
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 1–9.
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411.
- Xu, C.; Zhang, W.; Tang, J.; Pan, C.; Yu, G. Porous Organic Polymers: An Emerged Platform for Photocatalytic Water Splitting. Front. Chem. 2018, 6, 592.
- Evans, A.M.; Ryder, M.R.; Ji, W.; Strauss, M.J.; Corcos, A.R.; Vitaku, E.; Flanders, N.C.; Bisbey, R.P.; Dichtel, W.R. Trends in the thermal stability of two-dimensional covalent organic frameworks. Faraday Discuss. 2021, 225, 226–240.
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Fan, H.; Wang, X.; Zhang, C.; Zhang, Y. Engineering approach toward catalyst design for solar photocatalytic CO2 reduction: A critical review. Int. J. Energy Res. 2021, 45, 9895–9913.
- Wang, H.N.; Zou, Y.H.; Sun, H.X.; Chen, Y.; Li, S.L.; Lan, Y.Q. Recent progress and perspectives in heterogeneous photocatalytic CO2 reduction through a solid–gas mode. Coord. Chem. Rev. 2021, 438, 213906.
- Pang, R.; Teramura, K.; Tatsumi, H.; Asakura, H.; Hosokawa, S.; Tanaka, T. Modification of Ga2O3 by an Ag-Cr core-shell cocatalyst enhances photocatalytic CO evolution for the conversion of CO2 by H2O. Chem. Commun. 2018, 54, 1053–1056.
- Pham, T.D.; Lee, B.K. Novel photocatalytic activity of co-doped TiO2/PU for CO2 reduction with H2O vapor to produce solar fuels under visible light. J. Catal. 2017, 345, 87–95.
- Xin, C.; Hu, M.; Wang, K.; Wang, X. Significant Enhancement of Photocatalytic Reduction of CO2 with H2O over ZnO by the Formation of Basic Zinc Carbonate. Langmuir 2017, 33, 6667–6676.
- Li, D.; Ouyang, S.; Xu, H.; Lu, D.; Zhao, M.; Zhang, X.; Ye, J. Synergistic effect of Au and Rh on SrTiO3 in significantly promoting visible-light-driven syngas production from CO2 and H2O. Chem. Commun. 2016, 52, 5989–5992.
- Wang, Y.; Xu, Y.; Wang, Y.; Qin, H.; Li, X.; Zuo, Y.; Kang, S.; Cui, L. Synthesis of Mo-doped graphitic carbon nitride catalysts and their photocatalytic activity in the reduction of CO2 with H2O. Catal. Commun. 2016, 74, 75–79.
- Thanh Truc, N.T.; Giang Bach, L.; Thi Hanh, N.; Pham, T.D.; Thi Phuong Le Chi, N.; Tran, D.T.; Nguyen, M.V.; Nguyen, V.N. The superior photocatalytic activity of Nb doped TiO2/g-C3N4 direct Z-scheme system for efficient conversion of CO2 into valuable fuels. J. Colloid Interface Sci. 2019, 540, 1–8.
