The enteric nervous system (ENS) is a complex network of neurons and accompanying glial cells (enteric glial cells, EGCs) which controls the major functions of the gastrointestinal (GI) tract.
At first, glia were considered to be just a structural support for neurons, but recent findings emphasized more on their functions, and they turned out to be equally as important as neural cells, due to their involvement in all aspects of neural functions for both the central and peripheral nervous system, including the ENS.
In this review, we focus on the enteric glia, their role and functions in physiology and pathology as well as the available studies on the effects of different nutraceuticals as modulators of these interesting cells.
2. Enteric Glial Cells
The ENS is a network of neurons divided into submucosal and myenteric plexuses, together with their accompanying glia, the EGCs [
2]. Originally, EGCs were considered as a structural support for the enteric neurons, however recently, it was proved that they are crucial for the functioning of the GI tract under physiological (intestinal barrier support, GI motility, sensation) and pathophysiological conditions (GI motility disturbances, visceral pain).
Hanani et al. distinguished and classified EGCs into four subgroups based on their morphology (
Table 1) [
3].
Abbreviations: EGC, enteric glial cell. Created in
biorender.com (accessed on 15 March 2021).
Besides morphology, EGCs may also be classified according to the molecular or functional differences in receptors or channels expressed on their surface or in their nuclei. The following proteins are currently used to identify EGCs, i.e., calcium-binding protein S100β [
4], glial fibrillary acidic protein (GFAP) [
5] and the transcription factors: SOX8, SOX9, SOX10 [
6].
As shown in Table 2, EGCs share some similarities with astrocytes, the major type of glial cells of the central nervous system.
Table 2. Comparison between enteric glial cells and astrocytes [
6,
7,
8].
| Feature |
Enteric Glial Cells |
Astrocytes |
| Morphology |
Irregularly branched processes |
In vivo: numerous processes forming well-delineated bushy territories
In culture: few processes, polygonal fibroblast-like shape
Astrocytes show structural plasticity: their morphology differs between brain areas, and it may be changed (stellation or process growth) by different stimuli |
| Subtypes |
Protoplasmic
Fibrous
Mucosal
Intermuscular |
Protoplasmic
Fibrous |
| Location |
Enteric nervous system (submucosal and myenteric plexus) |
Central nervous system |
| Identification |
GFAP
Calcium-binding protein S100β
Transcription factors (SOX8, SOX9, SOX10) |
GFAP
Calcium-binding protein S100β
Glutamine synthase
CD44
Vimentin
Ran-2
Astrocytes from different brain regions can exhibit pronounced molecular differences |
| Adjacent cell coupling |
Gap junction coupling |
Gap junction coupling |
| Activation |
Release of pro-inflammatory cytokines (i.e., IL-1β, TNF-α)
Increased expression of c-fos, TrkA, ET-B, TLR-4, BR1
Enhanced expression of glial cell markers |
Release of pro-inflammatory cytokines (i.e., IL-1β, IL-6, TNF-α, TGF-β)
Increased expression of adhesion-related molecules (CD44)
Increased expression of receptors for EGF, TNF-α
Enhanced expression of glial cell markers: GFAP, vimentin, nestin |
| Involved in |
Physiopathological modulation of GI functions |
Development and plasticity of dendritic spines and synapses
Elimination of dendritic spines,
synapse formation
Regulation of neurotransmission and plasticity |
Abbreviations: BR1, bradykinin receptor 1; CD44, membrane glycoprotein; EGF, epidermal growth factor; ET-B, entothelin-1 receptor B; GFAP, glial fibrillary acidic protein; GI, gastrointestinal; IL, interleukin; Ran-2, rat neural antigen-2; TLR-4, Toll-like receptor 4; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; TrkA, nerve growth factor receptor.
2.1. Cellular and Tissular Roles of EGCs
Generally, EGCs are considered as non-excitable cells, as they are unable to generate an action potential. Furthermore, EGCs are interconnected and electrically coupled by gap junctions that form an extensive glial network [
9], as shown in
Figure 1.
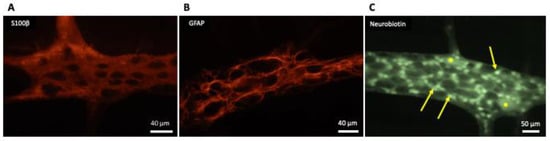
Figure 1. Appearance of enteric glial cells (EGCs). (
A,
B) Show the images obtained from the myenteric plexus of the rat distal colon; immunoreactivity to S100-β (
A) and glial fibrillary acidic protein (GFAP) (
B) are characteristic for EGCs. (
C) Shows the network of electrically-coupled EGCs (arrows) in one myenteric ganglion from the guinea pig ileum; this image was taken as the result of the accidental insertion of an electrode filled with neurobiotin in one EGC while performing electrophysiological recordings of the activity of myenteric neurons (*); neurobiotin injected into one EGC diffused throughout the gap junctions connecting it with the other EGC in the myenteric ganglion, in the same way as firstly described by Hanani et al. [
9] in 1989 for Lucifer yellow dye.
Enteric glial cells communicate with surrounding cells (neurons, glia, epithelial cells, immune cells) and integrate received information through calcium signaling [
10]. Intercellular communication is a result of the propagation of calcium waves through connexin 43 (Cx43) hemichannels [
11]. Moreover, EGCs are susceptible to the activation by neural pathways: intrinsic (from enteric neurons) or extrinsic (from autonomic or primary afferent neurons). The major neurotransmitter involved in this extracellular signaling is adenosine triphosphate (ATP) [
12]. It was found that intermuscular EGCs express the purinergic receptor (P2X7) [
13].
Like neurons, EGCs may release neurotransmitters and express the receptors for neurotransmitters on their surface to receive signals [
13,
14,
15,
16]. In particular, human EGCs were found to be immunoreactive to glutamate [
17] and gamma amino butyric acid (GABA) transporter (GAT2) [
18,
19]. Furthermore, EGCs exhibit immuno-reactivity for L-arginine, a nitric oxide (NO) precursor and thus they may be involved in nitrergic neurotransmission [
20,
21].
Interestingly, EGCs are characterized by displaying a remarkable function: they may be activated upon stimulation (e.g., inflammation or following the injury), and switched into a reactive, pro-inflammatory phenotype [
22,
23]. When EGCs are activated, they have an increased ability to proliferate [
24], enhance c-fos expression, and change their expression of markers and surface receptors [
25]. For example, the expression of nerve growth factor (NGF) receptor, tropomyosin receptor kinase A (TrkA) [
26], endothelin-1 receptor B (ET-B) [
27], Toll-like receptor (TLR) 4 [
28], and bradykinin receptor 1 (BR1) [
29] are increased in enteric glia incubated with interleukin-1β (IL-1β), and TrkA receptor is up-regulated in response to lipopolysaccharide (LPS) stimulation [
26].
Consequently, reactive glial cells are characterized by an increased expression of enteric glial markers. For instance, the expression of GFAP may be induced by the incubation with tumor necrosis factor α (TNF-α), IL-1β, LPS or LPS + interferon γ (IFN-γ) [
27,
30,
31]. The latter also increases the expression of S100β [
32]. In vivo, increased GFAP expression in the rat myenteric plexus occurred in LPS-induced intestinal inflammation [
33].
Moreover, reactive EGCs are able to release neurotrophins, growth factors or cytokines and therefore enteric glia recruit immune cells (macrophages, neutrophils, mast cells) into the colonic mucosa [
34,
35,
36]. This confirms an important immunomodulatory role for these cells within the GI tract.
The EGCs that are located directly underneath the epithelial layer constitute a link between the epithelium and submucosal neurons, and they participate in all steps of epithelial regeneration (cellular differentiation, migration, adhesion and proliferation) [
37]. Therefore, EGCs support the epithelial barrier integrity in the intestines and have the capacity to enhance epithelial healing. Glial cell derived neurotrophic factor (GDNF) released by EGCs entails anti-inflammatory effect in the intestines through the inhibition of cellular apoptosis and decrease of pro-inflammatory cytokine level [
35,
38]. Furthermore, during mild inflammation, GDNF helps in the processes of epithelial reconstitution and maturation [
39]. In addition, EGCs produce and release several factors involved in the processes of epithelial regeneration: pro-epithelial growth factor (pro-EGF) [
40]. S-nitrosoglutathione [
41] or 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) [
42]. EGCs support the intestinal barrier through decreasing intestinal permeability [
41] or increasing the resistance to infections [
43].
Enteric glial cells are also involved in the control of GI motility, as they coordinate sensory and motor signaling within the GI tract [
44]. Noteworthily, according to Aubé et al. [
45], a progressive loss of EGCs in transgenic mice, expressing haemagglutinin (HA), that received activated HA specific CD8+ T cells, led to the prolongation of the GI transit. In the study by Nesser et al. [
46], in mice treated with fluorocitrate, a selective gliotoxin, the upper GI transit time was prolonged and the intestinal motility patterns were impaired (both the basal tone and the amplitude of contractility in response to electrical field stimulation were decreased).
Finally, ECGs are considered to be involved in visceral sensation, via directly or indirectly sensitizing or activating nociceptors. Additionally, EGCs have the potential to regulate nociceptor sensitization/activation by removing of neuromodulators [
47]. Direct mechanisms of sensitization include the release of neuromodulators such as ATP, GABA, IL-1β and neurotrophins. Indirect mechanisms involve antigen presentation through major histocompatibility complex (MHC) class I and II, leading to activation of T cells followed by cytokine release, and regulation of other immune cells, leading to release of histamine and further cytokines (TNF-α, IL-1β) [
47]. Moreover, pro-inflammatory signals induce glial Cx43-dependent macrophage colony-stimulating factor (M-CSF) production through protein kinase C (PKC) and TNF-α converting enzyme (TACE). This further supports the importance of EGC interaction with macrophages in the regulation of visceral hypersensitivity during chronic inflammation [
48].
2.2. Physiological Changes in the Population of EGCs
The population of EGCs may be altered by many physiological factors, such as aging or diet modifications. The process of aging of the GI tract includes a progressive loss of EGCs. Philips et al. [
49] compared the population of EGCs in the GI tract in young (5–6 months-old) and old (26-month-old) rats. According to their findings, there was a significant decline in the number and density of EGCs in the myenteric plexus from the duodenum up to the distal colon with age. However, there was a small, non-significant decrease of EGC number in the rectum.
Interestingly, more detailed research revealed that diet also influences the population of EGCs. A high-fat diet caused a significant loss of EGC density in duodenal submucosal plexus in mice [
50]. On the contrary, the same diet increased the number of EGCs in the myenteric plexus of the antrum, while it remained unchanged in the jejunum [
51]. In contrast to the alterations in the enteric glia, high-fat diet led to a substantial loss of myenteric neurons, while the population of submucosal neurons stayed within the norm [
52].
On the other hand, food restrictions that slow the aging process by reducing the oxidative processes thus inhibiting of cell death, turned out to be detrimental for the EGCs. According to the study by Schoffen et al. [
53], diet restriction accentuated morphologic and quantitative changes in glial cell populations in rats, whereas the 50% reduction of food supply entailed neuroprotective effects on the myenteric neurons in the colon.
Nevertheless, the mechanisms responsible for the gliopathy occurring with age or diet modifications remain unknown. It is yet to be clarified whether the changes in morphology or number of EGCs are due to a direct impact of aging/diet restrictions or rather a consequence of the concomitant degenerative processes of the neurons in the ENS.
3. Role of EGCs in GI Pathophysiology
As EGCs coordinate the communication between the cells in the GI tract (neurons, epithelial cells, myocytes), any alterations in their population (such as those associated with the occurrence of different diseases) may have a significant impact on the GI functions.
3.1. Intestinal Inflammation
Inflammatory bowel disease (IBD) is a group of chronic inflammatory conditions of the GI tract and two major types, Crohn’s disease (CD) and ulcerative colitis (UC) are distinguished. The first reports regarding the importance of enteric glia in the inflammatory processes in the GI tract came from 1998. Bush et al. [
54] generated transgenic mice through the ablation of GFAP-positive glial cells from the jejunum and ileum, resulting in fulminating and fatal jejuno-ileitis. The ablation of EGCs led to severe inflammations, causing degeneration of neurons in the ENS and hemorrhagic necrosis of the small intestine. The alterations within the gut were similar to the pathology in the course of IBD in both animals and humans [
54]. Consequently, the concept about the involvement of EGCs in the inflammatory processes in the GI tact emerges.
Noteworthily, Pochard et al. [
25] summed up the results of molecular studies on the population of EGCs in IBD: in most studies, the expression of GFAP, S100β and GDNF was elevated in inflamed colon of IBD patients (both CD and UC) in comparison to their healthy colonic tissue [
55,
56,
57]. The expression of GFAP was decreased in healthy intestinal samples from CD patients [
55,
56], but not UC, comparing to healthy patients. The expression of S100β was downregulated in the myenteric plexus of uninflamed areas from CD patients in comparison to healthy controls [
57]. Likewise, in the rectum of UC patients the submucosal expression of S100β was decreased in comparison to healthy controls [
23,
30]. Noteworthily, GDNF production was increased in samples collected from healthy parts of the colon of UC patients as compared to healthy controls [
56]. Interestingly, GDNF ameliorated experimental colitis, inhibited mucosal inflammatory response and decreased intestinal permeability in the mouse model of colitis induced by dextran sodium sulfate (DSS) [
58].
The differences in the expression of glial markers in the course of IBD do not reflect the extent of alterations in the population of EGCs in the intestines during inflammation. The decreased expression of GFAP, located in the cytoplasm of CD patients may be considered as a sign of glial loss, but GFAP immunohistochemical staining is not optimal to quantify the number of cells. The emerging approach, that could possibly be used for further assessment of the enteric glia population in the course of IBD is the utilization of proteins located in the nucleus (such as SOX 8/9/10) [
59].
Besides the potential glial loss in the course of IBD, the functional differences appear to be significant. Coquenlorge et al. [
60] assessed that, although EGCs isolated from controls and CD patients exhibited similar expression of glial markers (GFAP, S100β) and EGC-derived factors (IL-6, TGF-β, pro-EGF and glutathione (GSH)), they differed in their influence on the intestinal barrier. Enteric glial cells from CD patients failed in supporting the intestinal barrier and the healing process opposite to those from healthy controls. This study was further expanded on the UC patients. It assessed how EGCs isolated from UC patients affect epithelial barrier of the intestines. It was confirmed that, unlike CD patient derived EGCs, EGCs from UC patients preserve intestinal permeability. The efficiency of the intestinal barrier was similar in co-culture with EGCs derived from UC patients and healthy controls [
25].
Under physiological conditions, MHC class I receptors are expressed on the enteric glia, while MHC class II remain almost undetectable [
61,
62]. However, after the exposure to enteroinvasive Escherichia coli, the expression of MHC class II on the enteroglial cells is increased [
63]. Moreover, the expression of MHC class II was significantly increased in CD patients in comparison to healthy controls, in which the expression of these receptors was very low or even absent [
61,
62].
3.2. Chronic Constipation
Chronic constipation is a condition characterized by a lack of frequent bowel movements or difficulties of stool passage. Chronic constipation may be related to the organic barriers in the colon or rectum (i.e., tumor), neuronal/muscular impairment (i.e., dysmotility in Parkinson’s disease), post-infection (megacolon caused by Chagas disease) or idiopathic (idiopathic constipation). The results of clinical studies on the importance of EGCs in the control of GI motility indicate that a loss of enteric glia in the ENS may be associated with dysmotility (i.e., idiopathic constipation or infectious-related dysmotility).
According to Bassotti et al. [
64], who examined patients with constipation and collected samples from the ileum and colon, there was a loss of EGCs in these tissues. Notably, the decrease in the number of EGCs was accompanied by the reduction of enteric neurons density. Similar results were obtained in a group of patients with severe, intractable constipation that underwent colectomy with ileorectostomy, as they displayed a significant decrease in neurons, EGCs and interstitial cells of Cajal. Constipated patients had significantly more apoptotic enteric neurons in comparison to controls [
65].
Noteworthily, the population of EGCs in the submucosal and myenteric plexus was significantly decreased in the colon of patients with severe constipation due to obstructed defecation refractory to medical treatment or biofeedback training. At the same time, the enteric neurons were reduced only in the submucosal plexus [
66].
In the case of megacolon occurring in the course of Chagas disease (an infectious disease caused by Trypanosoma cruzi) and idiopathic megacolon, there was a remarkable reduction in the number of neurons and EGCs in the ENS in the colonic specimens collected during surgery. However, the differences in the population of EGCs were more pronounced in the group of patients with infectious megacolon [
67].
3.3. Postoperative Ileus
Postoperative ileus (POI) is a condition that may occur after surgery of the abdominal cavity or the outer abdomen, which is associated with GI motility impairment and results in inhibition of peristalsis and distension. Although the pathophysiology of POI remains unknown, recent studies indicate that EGCs maintain an important role in this process. Stoffels et al. [
68] investigated the molecular mechanism of POI in mice. They determined that the blockage of the receptor for interleukin 1 (IL-1R) attenuated the POI. These receptors were found to be expressed on the surface of EGCs in the myenteric plexus. The activation of IL-1R in cultured EGCs promoted an inflammatory response through an increase in IL-6 and monocyte chemotactic protein 1 (MCP-1) levels, which may be an important step in the development of POI.
3.4. Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is a chronic disease of the GI tract that manifests with recurrent abdominal pain accompanied by GI motility disturbances. This functional GI disorder may be classified as diarrhea-predominant IBS (IBS-D), constipation-predominant (IBS-C) or mixed IBS, when both diarrhea and constipation occur in an alternate manner (IBS-M).
According to Lilli et al. [
69] the immunoreactivity of S100β was significantly reduced in the colonic biopsies of IBS patients, independently of the IBS subtype (IBS-C, IBS-D, and IBS-M). Furthermore, the incubation of the rodent EGCs with supernatants from the mucosal biopsies from IBS-C patients reduced the cellular proliferation. Noteworthily, exposure of rat enteric glia with IBS-D and IBS-M supernatants impaired ATP-induced Ca
2+ response of these cells.
In some cases, one more type of IBS can be distinguished: the one followed by the bacterial, viral or parasitic infection of the GI tract (post-infectious IBS, PI-IBS) [
70,
71]. Notably, there were many attempts to elucidate the molecular mechanism that underlies PI-IBS, for example: hyperplasia of enterochromaffin cells, increased intestinal permeability or enhanced cytokine production [
72,
73]. Importantly, one of the proposed mechanisms of PI-IBS followed by Clostridium difficile infection involves EGCs. Toxin B produced by C. difficile evokes cytotoxic and pro-apoptotic effects on EGCs in vitro. This harmful impact of toxin B on enteric glia results from the disorganization of cytoskeleton, early cell rounding with Rac1 glucosylation, cell cycle inhibition and increased susceptibility to apoptosis induced by the pro-inflammatory cytokines (TNF-α and IFN-γ). Importantly, despite these direct effects of toxin B, it is important that EGCs which survive the detrimental action of toxin B, do not recover and their function is not restored (they exhibit persistent Rac1 glucosylation, disturbances in the cell cycle and low apoptosis rate) [
74]. The long-term effects of C. difficile infection on EGCs network may be pivotal for GI homeostasis, as enteric glia coordinate cell-to-cell communication in the intestines [
75].
The severity of visceral hypersensitivity in IBS patients may be associated with brain derived neurotrophic factor (BDNF), a protein described as crucial in the process of neuropathic and inflammatory pain. The level of BDNF was significantly elevated in the colonic mucosal biopsies from IBS patients and corresponded with the abdominal pain severity [
76,
77]. The high-affinity receptor for BDNF, tropomyosin receptor kinase B (TrkB), is expressed on the surface of EGCs [
77]. Interestingly, the expression of this receptor, along with GFAP and substance P (SP), was increased in the colonic mucosa of IBS patients. It suggests that BDNF may play a key role in the occurrence of visceral hypersensitivity, i.e., in the course of IBS, through the interactions with EGCs. It was determined that the administration of fecal supernatants from IBS-D patients failed to induce visceral hypersensitivity in BDNF ± mice in contrast to wild type animals. In wild type animals, the pain threshold to colorectal distension after IBS-D fecal supernatant administration was significantly elevated when pretreated with a TrkB antagonist (TrkB/Fc). Noteworthily, the induction of visceral hypersensitivity evoked the up-regulation of the same proteins (TrkB, GFAP, SP) as in IBS patients in wild type animals, but not in the BDNF ± mice [
78]. Overall, the fecal supernatant from IBS patients induced hypersensitivity that may involve a BDNF-TrkB signaling pathway. Thus, BDNF appear to act as a link between visceral hypersensitivity and EGC activation.
The first step of the non-pharmacological management of IBS is diet modification. A dietary approach may involve the consumption of a low FODMAP products (diet low in fermentable carbohydrates). It was assessed that IBS patients have a higher Firmicutes/Bacterioidetes ratio and bacteria from the phylum Firmicutes are considered as a major source of the short chain fatty acid butyrate, a small molecule metabolite arising from symbiotic bacteria fermentation from insoluble dietary fibers [
79,
80]. The lower supply of fermentable carbohydrates alleviates IBS symptoms [
81]. Furthermore, the butyrate enemas induce visceral hypersensitivity in animals tested. It was elucidated that butyrate-induced hypersensitivity is associated with the up-regulation of NGF on messenger ribonucleic acid (mRNA) and protein level thus EGCs are one of the major sources of NGF in the GI tract. Noteworthily, NGF was co-expressed with GFAP and the co-localized immunostaining area of NGF and GFAP was increased in the colon of rats that received butyrate-enema. Furthermore, it was reported that the secretion of NGF from EGCs in the colonic lamina propria was increased after the butyrate-enema [
82].
3.5. EGC and Pathophysiology Outer the GI Tract
Intestinal motility disfunction may also be a characteristic symptom of diseases outer the GI tract, for example neurodegenerative diseases, such as Parkinson’s disease (PD) or prion diseases. PD is a long-term, multi-system disease of the CNS, which is related to degeneration of the dopaminergic neurons. Besides the motor symptoms (rigidity, tremor, dyskinesia), the intrinsic aspect of PD is a dysfunction of the GI tract. Patients experience nausea, dysphagia, abdominal distension and constipation. Studies show that, in the colon of PD patients, there was an increased expression of glial markers (GFAP, S100β, SOX10), and this was accompanied by the elevation of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, IL-6) at the mRNA level. However, there was no correlation found between the expression of glial markers or the inflammatory indicators and the severity of disease or GI symptoms [
83]. Likewise, according to Clairembault et al. [
84], in the colonic biopsies from PD patients, there was a GFAP over-expression and a reduction in GFAP phosphorylation comparing to healthy controls. These results suggest that EGCs may be involved in the GI dysfunction observed in the course of PD, nevertheless further research is needed to understand the mechanism of this process.
Prion diseases are progressive and fatal neurodegenerative conditions which are caused by spreading of pathological isoforms of cellular prion protein. This pathological process affects astrocytes in the CNS and EGCs in the GI tract [
85,
86]. However, it was assessed that the prion replication sites were found in the ENS prior to the replication in the CNS [
87]. Thus, the enteric glia may be essential in prion neuroinvasion, as the GI system constitutes to the major exogenous prion protein entry site and acts as the starting point for the prions en route to the brain [
87].
Finally, many systemic diseases may cause alterations in the GI function inducing many effects on the EGCs. For example: diabetes [
88] or autoimmune diseases such as rheumatoid arthritis [
89]. Interstingly, some dietary components have proved beneficial in protection against EGC alterations in those diseases, thus favoring the restoration of GI altered functions.