Albumin is a versatile protein being used widely for developing carriers for drugs and nucleic acids. It provides biocompatibility, tumor specificity, the possibility for surface modification, and reduces toxicity.
- albumin
- gene therapy
- cancer
- nanocarriers
- surface modification
1. Introduction
2. Nucleic Acids in Cancer Therapy
3. Albumin-Based Nanocarriers
3.1. Albumin
3.2. Albumin in Cancer Therapy
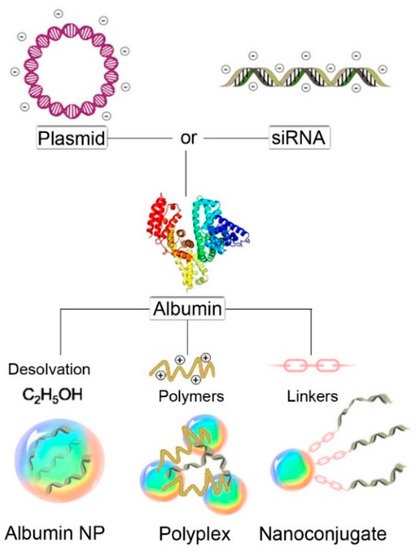
3.3. Albumin Nanocarrier for Gene Therapy in Cancer
|
Therapeutic Nucleic Acid |
Type of Nanocarrier |
Size (nm) |
Z-Potential (mV) |
Model System |
|---|---|---|---|---|
|
Plasmid |
||||
|
Plasmid pORF-hTRAIL (pDNA) |
BSA NPs |
115.7 |
−15.4 (pH 7) +11.3 (pH 2) |
BALB/c mice bearing i.c. C6 gliomas (Brain Tumor [23]) |
|
Plasmid pCMV-EGFP-C |
PEI Polyplex |
140–450 |
NA |
HeLa cells [44] |
|
hMDA-7 plasmid |
BSA NPs |
115.6 |
+33.8 |
PANC-1 and BXPC-3 human pancreatic cell lines and tumor-induced BALB/c nude mice [24] |
|
pGL3 vector coding for the firefly luciferase gene |
HSA-PEI NPs |
300 to 700 |
−7 in H2O +16 in 1 mM KCl |
Human epithelial kidney 293-cells [45] |
|
Oligonucleotides |
||||
|
Oligonucleotide |
Nanoconjugate |
13 |
NA |
Tumor spheroids of A375/GFP cells [46] |
|
Antisense Oligonucleotides (ASOs) |
HSA NPs |
290–330 |
NA |
MCF-7 cells [47] |
|
Akt1 ASOs |
Lipid-HSA NPs |
108.6 |
10.5 |
KB cells and A549 cells [48] |
|
siRNAs |
||||
|
VEGF siRNA |
Self-crosslinked HSA NPs |
169.3 |
NA |
B16F10 murine melanoma cells, squamous cell carcinoma cells (SCC7), and human prostatic carcinoma cells (PC-3) [49] |
|
Bcl-2-specific siRNA |
Anti-ErbB-2 antibody conjugated BSA nanocomplex |
278 |
−39.6 |
SK-BR-3 and MCF-7 breast cancer cells [50] |
|
phrGFP-targeted siRNA |
HSA-coated lipid NPs |
79.5 |
+15.3 |
MCF-7, MDA-MB-231, SK-BR-3 cells, and phrGFP-transfected MCF-7 xenograft tumor mice model [51] |
|
Immunotherapeutic biologics |
||||
|
Vaccine conjugated with Evans blue (EB) and CpG |
Albumin/vaccine nanocomplexes |
~13 |
NA |
Female C57BL/6 mice s.c. inoculated with EL4 cells, or EG7.OVA cells, B16F10 cells, MC38 cells on the shoulder [52] |
|
PD-L1 plasmid (CRISPR/Cas9) |
Stearyl PEI complexed HSA NPs |
203 |
13 |
Mouse colon carcinoma CT26 cells [53] |
BSA NPs = Bovine Serum Albumin Nanoparticles; HAS = Human Serum Albumin; VEGF = Vascular Endothelial Growth Factor; CRISPR = Clustered, Regularly Interspaced, Short Palindromic Repeat.
3.4. Albumin Nanoparticles
3.5. Polyplexes
3.6. Nanoconjugates
4. Albumin as a Coating Agent
5. Nucleic Acid-Loaded Albumin Nanocarriers for Immunotherapy
6. Conclusion
In conclusion, albumin nanocarriers have been studied widely for gene therapy in cancer because of the unique features of albumin, such as the ease of preparation, high stability, and biocompatibility. Furthermore, the surface of those nanocarriers can be modified to enhance the therapeutic efficiency and selectivity, whereas reducing the undesired off-target effects. Despite all those features, some limitations are still being reported and need to be addressed properly, such as the albumin catabolism, which may be affected by various factors such as the levels of corticosteroids. Therefore, further studies are required to ensure the safe use of those nanocarriers to ease their way to the clinic.
In addition to the prevalent conventional gene therapy, which is mainly focused on the expression of a DNA fragment or its random insertion into the genome, various specific gene-editing tools such as CRISPR/Cas9 have been introduced. These gene-editing tools have promising potential for the introduction of personalized medicines in cancer therapy. In a similar way, novel nucleic acid-based therapies such as chimeric antigen receptor (CAR) T approaches are being developed as a promising therapeutic approach in immuno-oncology. The combination of the advantages imparted by the albumin-based nanocarriers with powerful therapies including CRISPR/Cas9 and CAR-T will revolutionize the treatment options in oncology. Though there are limited studies available on the incorporation of these gene-editing tools in albumin nanostructures, the profound therapeutic application of these vectors is on the near horizon.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13143454
