A novel approach in livestock production is applying nanominerals, especially selenium (Se) and zinc (Zn), which can serve as a platform to incorporate these elements into the body. This approach enables direct transportation of active compounds to target organs, avoiding their fast degradability and encouraging several health benefits.
- nanominerals
- manufacturing
- bioavailability
- hazards
- health
- livestock
1. Introduction
A novel approach in livestock production is applying nanominerals, especially selenium (Se) and zinc (Zn), which can serve as a platform to incorporate these elements into the body. This approach enables direct transportation of active compounds to target organs, avoiding their fast degradability and encouraging several health benefits [1][2]. So far, studies have shown that the application of nanominerals in the production, immunity, and reproduction of animals is promising [3][4], but adverse effects and toxicity are also reported [1][2]. Inclusion of different types of selenite (sodium selenite, selenized yeast, and elemental nano-Se) increased Se levels in whole blood, serum, and tissue.
Many beneficial impacts of nano-Zn have been reported, such as production-promoting, enhancing animal reproductive efficiency, and antibacterial and immunomodulatory properties. Nano-Zn oxide treatment has been identified in cows, milk value has increased in clinical mastitis, milk yield has been suppressed by nano-Zn intake in dairy animals, and subclinical mastitis has been suppressed (reduced somatically counting) [3][2][5][6][7][8][9][10][11][12][13].
2. Preparation of Nanominerals
In the livestock sector, the nutritional values of feed can be enhanced by using organic nanoparticle (such as proteins, fats, and sugar molecules) supplementation [14]. Nutrients, in the form of nanoparticles, can be encased as nanocapsules and transported through the gastrointestinal tract (GIT) into the bloodstream, and then into many body organs, such as the brain, liver, kidney, heart, stomach, intestine, and spleen, multiplying the bioavailability of the delivered nutrients [6][7]. As for inorganic nanoparticles, minerals have been used widely as nanoparticles such as calcium, magnesium, silicon dioxide, and silver nanoparticles in water and animal-related [15][9][10][11]. There are many manufacturing methods used for nanomineral fabrication with different physicochemical properties [12][13].
The intrinsic properties of nanominerals are generally determined by their shape, size, crystalline structure, composition, and morphology [16]. The shapes of the nanoparticles are numerous, including spheres, cones, rolls, worms, rectangular discs, canes, and circular or elliptical discs. Many factors that affect nanoparticles’ effectiveness, such as thickness and viscosity, are the base fluid viscosity, amount of nanoparticles, shape, type, diameter of particles, type, pressure, temperature, shear rate, and pH value [17][18]. Moreover, nanoparticles may show regions with several curvatures, texture concavity, and other properties that critically impact the adhesion strength that affects the effectiveness of the delivery and the efficacy of nanoparticles [6][7][16][17][18].
The notable characteristics of nanominerals are determined mainly by their shape, size, crystalline aspects, composition, morphology, and structure. Functional activities (catalytic, chemical, or biological impact) of nanominerals are strongly affected by their particles’ sizes [16]. Nanominerals have large surface areas, allowing better interface with other organic and inorganic constituents.
So, the creation of some sensitive and practical approaches to synthesize the desired nanoparticle is required. During the initial stages of nanoparticle synthesis, the main aim was to have a preferable hegemony over particle size, purity, morphology, quality, and amount [17]. As a result, different methods have been approved to synthesize nanoparticles, such as chemical, physical, and biological processes (Figure 1). In this section, we will describe the advantages and disadvantages of these techniques.
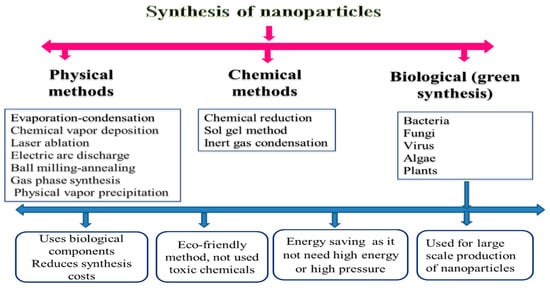
According to many previous investigations, there is a broad framework of physical approaches for nanoparticle preparation. For instance, these approaches are similar to evaporation–condensation, which takes place by applying a boiling tube at atmospheric pressure [19]; ablation, which takes place by laser and evaporation–condensation together [18]; chemical vapor deposition; electric arc discharge; ball milling–annealing; gas-phase synthesis methods [8]; physical vapor precipitation [20]; etc. The inclusion of high energy and ball milling (HEBM) methods is quite effective (1000 times) for synthesizing the nanominerals more than traditional ball mills [21]. This processing technique, also called mechanochemical synthesis, has already been used to prepare various materials such as amorphous metallic alloys, composites, and the modification of different classes of inorganic materials [22].
Generally, a longer milling period is required for HEBM to stimulate and complete the structural alterations. However, controlled milling temperature and atmosphere must be monitored when using the HEBM. The shortfall in the synthesis of the gas phase of nanomaterials is that it usually leads to the deposition of particles with larger sizes (from 10 to 200 nm) The advantages of physical methods for the synthesis of nanominerals include the absence of solvent contamination and the maximal recovery of nanoparticles [8].
The chemical method represents a direct approach for synthesizing and producing materials, including several steps in the gas or liquid phase. First, the atoms’ formation can be achieved using chemical reactions under control. Thus, newly formed atoms can then undergo elementary nucleation followed by growth processes, leading to specific nanomaterials [23]. The use of chemical methods in the synthesis of nanoparticles is characterized by the extraction of nanomineral particles from modification, solvent, mass production, processing control, and stabilization of nanominerals particles from agglomeration, as well as the possibility of achieving effective and controlled bulk production compared with physical methods [24].
Chemical methods produce uniform and nanosized particles, but physical processes have an ample particle size range [25]. It is the most suitable for reducing the size of molecules [26]. Hence, there have been many attempts to use eco-friendly chemicals, fungal components, and plants in the production of nanomineral particles [27]. Otherwise, microwave synthesis and nonchemical methods are substitutes to toxic chemical methods for the creation of nanomineral particles in a cost-effective manner and large scale [8].
In chemical methods, surfactants, such as polyvinyl pyrrolidone, cyclodextrin, quaternary ammonium salts, or polyvinyl alcohol, and stabilizing agents are required to inhibit the agglomeration of the metal particles [28]. Stabilizers keep the produced nanomineral particle aggregation in check, prevent the reduction of uncontrollable particle size, control particle size, and allow solubility of particles in different solvents [29]. Solvent molecules can further stabilize nanomineral particles [30]. Ligands, such as amines, phosphine, carbon monoxide, thiol, etc., can be used as stabilizers in the production of nanomineral particles through the coordination between the metal nanomineral particles and the ligand moiety [24].
Bacteria, viruses, algae, and plants are now used in the production of nanoparticles due to their biological advantages (low cost, nontoxic, and energy efficient) [25][31]. Biological methods were successfully used in the synthesis of different metal molecules such as gold, silver, selenium, cadmium, barium titanate, titanium, and palladium by using various plant materials [32][33], while the biosynthesis of ZnO nanoparticles was previously prepared by usingParthenium hysterophorousleaves [31]. The use of plant materials in nanomineral synthesis is advantageous and easier as this process is safe and straightforward. Although there are many advantages to biological methods, there are limitations of these methods, such as maintaining the culture media, the culture condition, the difficulty in product recovery, and the period in the creation of the nanomineral particles [26].
Little is known about the potential synthetic methods used to produce Se nanoparticles. [34] reported the potential of usingAllium sativumextracts in the green synthesis of Se nanoparticles. The conventional chemically synthesized and green-fabricated Se nanoparticles were investigated to assess their cytotoxicity against Vero cells. The values of CC50 (cytotoxicity concentration 50%) indicate biologically synthesized Se nanoparticles show biocompatible features and decrease cytotoxicity compared with chemically synthesized Se nanoparticles.
3. Applications of Nanominerals in Ruminants
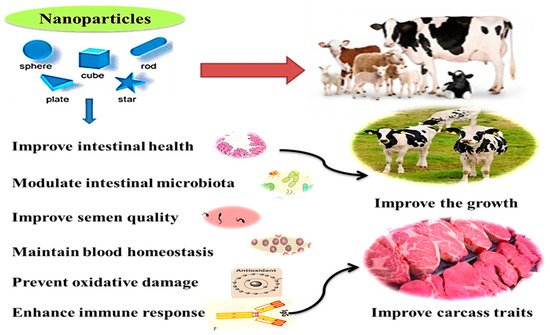
Microminerals can be useful for improving health and immunity, digestive system functions, microbiota homeostasis, metabolism, and reproductive performance in ruminants [35]. Additionally, they can be used for producing functional and safe animal products, maybe through eliminating the antibiotic use and increasing concentrations of trace minerals in animal products (meat and milk) required for better human health [14][3][4][1]. The health benefits and practical application of nanominerals in ruminants are illustrated in Figure 2. These effects will be displayed in detail in the following sections.
Recently, nano-ZnO nanoparticles (ZnNPs with size within 1–100 nm) are getting more attention for use in the mineral nutrition of livestock to address dietary requirements and to promote animal growth [20]. The inclusion of ZnNPs (100 and 200 mg/kg) has increased the volatile fatty acids, microbial crude protein, and degradation of organic matter at the 6th and 12th hours of incubation period under in vitro rumen fermentation conditions [36]. These positive effects are also seen in vivo with adult and/or growing animals; in ewes, the dietary supplementation of ZnNPs significantly increased the digestibility of DM, organic matter, nitrogen, and crude fiber-free extract compared with Zn larger particle and control ewes [37][38]. A positive effect has also been found in response to dietary supplementation with 50 mg Zn/kg DM, in the form of ZnO or nano-ZnO, on dry matter digestibility in Holstein calves [13].
Overall, these positive effects on rumen fermentation and nutrient digestibility could be ascribed to the increased surface area to volume ratio, nanoscale size, rapid and specific movement, and catalytic effectiveness. These contribute to improving absorption bioavailability of nanominerals in the GIT [39][40]. The enhancing effects of nanominerals on growth can be related to their ability to beneficially alter the gut microarchitecture of animals [41] and improve rumen fermentation, specifically fiber digestion and redox homeostasis [42][43][44].
Moreover, the semen purification and preservation processes have been established utilizing various nanomaterials and methods to obtain semen doses with high sperm quality [45]. During the freezing/cooling processes, sperm is preserved in synthetic extenders, which always need adjustment to maintain adequate semen quality traits. Accordingly, nanominerals have been utilized to modify semen extender properties, aiming to achieve antioxidant and antibacterial effects. Similarly, the dilution of semen with a zinc nano complex resulted in a higher activity of post-thawed sperm plasma membrane integrity with a better mitochondrial activity in a dose-dependent manner.
The affirmative result of ZnNPs and SeNPs administrations on semen traits could be owing to the possible role of these minerals as a co-factor in the activities of several antioxidant enzymes and the protective functions against reactive oxygen species [46][47]. Both Zn and Se have been recognized as favorable for the stability and viability of spermatozoa through avoiding protein degradation and inhabited enzymes, which leads to damaged DNA of spermatozoa [45][48]. In ART related to oocytes and embryos culture, the addition of appropriate levels of nanominerals to the culture media (in vitro maturation, in vitro fertilization, and embryo culture media) can improve the developmental competence of oocytes during in vitro maturation, as well as the fertilization rate, the cleavage rate and the quality of embryos [49]. However, compared to ART related to males, studies on the effects of nanominerals on the reproductive performance of female ruminants are too limited and require further exploring.
These microminerals play an indispensable role in spermatogenesis, sperm viability, sperm cell membrane integrity, and in maintaining the chromatin structure of sperm nuclei [48][50][49][51][52]. [53] found that oral administration of Zn oxide nanoparticles (ZnNPs) at a level of 80 ppm to rams significantly enhanced sperm motility and viability rates, semen volume, sperm concentration, and the functionality of sperm membrane by 20.96, 24.03, 33.9, 11.86, and 24.4%, respectively. Moreover, it also significantly reduced sperm morphological abnormalities by 28.3%, compared with the non-supplemented group. Studies have shown positive effects of mineral administrations during different reproductive windows in females [54][55].
Overall, the available literature highlights the positive roles of nanominerals on ART outcomes and on animals’ reproductive performance when supplemented with nanominerals. However, it is essential to note that the studies on in vivo models, either in males or females, are limited in drawing a complete overview for the effects of such additivies on reproductive performance and in knowing the dynamic mode of actions. The impacts of nano-Se and -Zn on the ruminant’s reproduction are found in Table 1.
| Element | Dose | Species | Major Effects |
|---|---|---|---|
| Nano-Se [56] | 0, 0.3, 3 and 6 g/kg DM diet fed for 75 days | Sheep (Dorset sheep × Small Tail Han × Tan sheep) | Nano-Se at 3 g/kg DM:
|
| Nano-Se and SS [57] | 1 mg/kg DM diet nano-Se and SS for 10 consecutive days | Sheep (Lori–Bakhtiari breed) | Nano-Se:
|
| Nano-Se [58] | 0.5 mg/kg DM diet nano-Se during gestation | Cashmere goat | Nano-Se:
|
| Nano-Se and SY [59] | 4 mg nano-Se and YS with 4 g Se-yeast | Sheep | Nano-Se:
|
| Nano-Se [60] | 0.1 mg/kg DM diet for 60 days | Sheep (neonatal lambs) | Nano-Se:
|
| Nano-Se, SS, and SY [61] | 0.3 mg/kg DM diet of nano-Se, SS and SY as compared to control (0.03mg/kg Se) | Taihang black goats | ADG was higher in Nano-Se and SY than SS or control group. Nano-Se:
|
| Nano-Se and SS [62] | 0.1 mg/kg live weight of nano-Se | Sheep (Makuei breed) | Nano-Se:
|
| Nano-ZnO and ZnO [38] | 30 or 40 mg/kg DM diet of nano-ZnO or ZnO for pre-partum and post-partum periods | Sheep (Khorasan-Kurdish breed) | Nano-ZnO:
|
| Nano-ZnO [63] | Iranian Angora goat | Nano-ZnO:
|
|
| Nano-ZnO [36] | 0, 50, 100, 200 or 400 mg/kg DM diet of nano-ZnO | In vitro ruminal fermentability |
|
| Nano-ZnO [13] | Cows exhibiting subclinical mastitis supplemented with 60 ppm inorganic zinc, zinc methionine, and nano-ZnO | Dairy cattle | Nano-ZnO:
|
| Nano-ZnO [64] | In vitro | Nano-ZnO:
|
Nanominerals may promote antioxidant activity by inhibiting the free radical’s production because of the increased surface area, leading to a higher number of active sites for scavenging an increased number of free radicals [54]. Sheep fed a basal diet containing ZnNPs exhibited a better antioxidant level [65][55]. Supplementation of SeNPs in newborn lambs promoted superoxide dismutase (SOD) levels with a concurrent reduction in thiobarbituric acid–reactive substances (TBARS) values [37].
The inclusion of Se NPs (0.6 mg Se head/day) in Khalkhali goat diets during the late stage of pregnancy significantly increased Se level in the blood (584.15μg/L) and serum (351.62 μg/L) of goats at kidding, compared with control goats (123.74 μg/L and 66.94 μg/L, respectively [55]. Moreover, it was found that introducing SeNPs to goats during the late stage of pregnancy significantly increased iron levels in the blood and serum of kids or goats and colostrum [66]. A better iron homeostasis capability was observed after the addition of SeNPs compared with other Se sources [67]. This may be due to the distinguished physicochemical features of SeNPs such as small size, large surface area, enhanced absorption via epithelial cells, and other functional properties.
The increase in blood antioxidant minerals is usually associated with particular improvements in the antioxidant status of animals and, thus, the health status of animals. SOD, catalase (CAT), and glutathione peroxidase (GSH-Px) compared with provided Se yeast and sodium selenite [61]. These antioxidant enzymes play the primary role of removing oxidative stress agents, such as malondialdehyde (MDA) and nitric oxide. [66] showed that SeNPs exhibited an excellent bioavailability due to their high catalytic efficiency, low toxicity, and absorbing solid ability.
Nanominerals also have protective effects against some physiological disorders; SeNPs showed a defensive impact on the cardiac cells from ischemia [54]. Additionally, due to the antibacterial activity of antioxidant nanominerals, some nanominerals, such as ZnO NPs, could be helpful in the prevention and curation of some bacterial-borne diseases such as sub-clinical mastitis in cows [13]. The impacts of nano-Se and nano-Zn on serum antioxidant parameters, immune response, and serum/milk Se contents in ruminants are summarized in Table 2.
| Element | Dose | Species | Major Effects |
|---|---|---|---|
| Nano-Se and SS [57] | Nano-Se and SS for 63 days | Sheep | Similar GSH-Px content in both sources of Se |
| Nano-Se, SS, and Se-Met [68] | 0.6 mg/head/d for 4 weeks before parturition | Pregnant goats | Nano-Se:
|
| Nano-Se [69] | 0, 1 and 2 mg/kg DM diet | Sheep (male Moghani lambs) | 2 mg/kg DM nano-Se:
|
| Nano-Se and SS [70] | 0.30 mg/kg of DM for one month | Dairy cows | Nano-Se:
|
| Nano-Se and SS [71] | 0.055 mg/kg BW for three months | Sheep (Lambs) | Nano-Se:
|
| Nano-Se [72] | 5 mg/kg BW/day | Wumeng semi-fine wool sheep | Nano-Se:
|
| Nano-Zn, ZnO, Zn-Met [73] | 28 mg/kg DM diet | Sheep | Nano-ZnO:
|
| Nano-ZnO and ZnO [36] | Nano-ZnO supplemented at 30 or 40 mg/kg DM for pre-partum and post-partum periods | Sheep (Khorasan-Kurdish breed) | Nano-ZnO:
|
This entry is adapted from the peer-reviewed paper 10.3390/ani11071916
References
- Fesseha, H.; Degu, T.; Getachew, Y. Nanotechnology and its application in animal production: A review. Vet. Med. Open J. 2020, 52, 43–50.
- Reddy, P.R.K.; Yasaswini, D.; Reddy, P.P.R.; Zeineldin, M.; Adegbeye, M.J.; Hyder, I. Applications, challenges, and strategies in the use of nanoparticles as feed additives in equine nutrition. Vet. World 2020, 13, 1685–1696.
- Huang, S.; Wang, L.; Liu, L.; Hou, Y.; Li, L. Nanotechnology in agriculture, livestock, and aquaculture in China. A review. Agron. Sustain. Dev. 2015, 35, 369–400.
- Osama, E.; El-Sheikh Sawsan, M.A.; Khairy, M.H.; Galal Azza, A.A. Nanoparticles and their Potential Applications in Veterinary Medicine. J. Adv. Vet. Res. 2020, 10, 268–273.
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141.
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901–11904.
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406.
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120.
- Bunglavan, S.J.; Garg, A.; Dass, R.; Shrivastava, S. Use of nanoparticles as feed additives to improve digestion and absorption in livestock. Livest. Res. Int. 2014, 2, 36–47.
- Al-Beitawi, N.A.; Shaker, M.M.; El-Shuraydeh, K.N.; Bláha, J. Effect of nanoclay minerals on growth performance, internal organs and blood biochemistry of broiler chickens compared to vaccines and antibiotics. J. Appl. Anim. Res. 2017, 45, 543–549.
- Raje, K.; Ojha, S.; Mishra, A.; Munde, V.; Chaudhary, S.K. Impact of supplementation of mineral nano particles on growth performance and health status of animals: A review. J. Entomol. Zool. Stud. 2018, 6, 1690–1694.
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686.
- Rajendran, D.; Kumar, G.; Ramakrishnan, S.; Shibi, T. Enhancing the milk production and immunity in Holstein Friesian crossbred cow by supplementing novel nano zinc oxide. Res. J. Biotechnol. 2013, 8, 11–17.
- Abd El-Hack, M.; Alagawany, M.; Farag, M.; Arif, M.; Emam, M.; Dhama, K.; Sarwar, M.; Sayab, M. Nutritional and pharmaceutical applications of nanotechnology: Trends and advances. Int. J. Pharmacol. 2017, 13, 340–350.
- Hashem, N.M.; El-Desoky, N.; Hosny, N.S.; Shehata, M.G. Gastrointestinal microflora homeostasis, immunity and growth performance of rabbits supplemented with innovative non-encapsulated or encapsulated synbiotic. Multidiscip. Digit. Publ. Inst. Proc. 2020, 73, 5.
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562.
- Allers, W.; Hahn, C.; Löhndorf, M.; Lukas, S.; Pan, S.; Schwarz, U.D.; Wiesendanger, R. Nanomechanical investigations and modifications of thin films based on scanning force methods. Nanotechnology 1996, 7, 346–350.
- Cardenas, G.; Meléndrez, M.; Cruzat, C.; Díaz, J. Synthesis of tin nanoparticles by physical vapour deposition technique (vd). Acta Microsci. 2007, 1, 1–2.
- Ingale, A.; Chaudhari, A. Biogenic Synthesis of Nanoparticles and Potential Applications: An Eco-Friendly Approach. J. Nanomed. Nanotechnol. 2013, 4.
- Swain, P.S.; Rajendran, D.; Rao, S.B.N.; Dominic, G. Preparation and effects of nano mineral particle feeding in livestock: A review. Vet. World 2015, 8, 888–891.
- Lane, R.; Craig, B.; Babcock, W. Materials engineering with nature’s building blocks. AMPTIAC Newslett. Spring 2002, 6, 31–37.
- Luz, M.S.D.; Tofanello, F.P.; Esposto, M.S.; Campos, A.D.; Ferreira, B.; Santos, C.A.M.D. Synthesis of SnSb2Te4 microplatelets by high-energy ball milling. Mater. Res. 2015, 18, 953–956.
- Yu, C.-H.; Tam, K.; Tsang, E.S.C. Chapter 5 Chemical Methods for Preparation of Nanoparticles in Solution. In Handbook of Metal Physics; Blackman, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 5, pp. 113–141.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Oremland, R.S.; Herbel, M.J.; Blum, J.S.; Langley, S.; Beveridge, T.J.; Ajayan, P.M.; Sutto, T.; Ellis, A.V.; Curran, S. Structural and spectral features of selenium nanospheres produced by se-respiring bacteria. Appl. Environ. Microbiol. 2004, 70, 52–60.
- Phan, H.T.; Haes, A.J. What Does Nanoparticle Stability Mean? J. Phys. Chem. C 2019, 123, 16495–16507.
- Szczepanowicz, K.; Stefańska, J.; Socha, R.; Warszyński, P. Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity. Fizykochem. Probl. Miner. 2010, 45, 85–98.
- Yang, J.; Deivaraj, T.C.; Too, H.-P.; Lee, J.Y. Acetate stabilization of metal nanoparticles and its role in the preparation of metal nanoparticles in ethylene glycol. Langmuir 2004, 20, 4241–4245.
- Zhou, Y. Recent Advances in Ionic Liquids for Synthesis of Inorganic Nanomaterials. Curr. Nanosci. 2005, 1, 35–42.
- Mary, E.; Inbathamizh, L. Green synthesis and characterization of nano silver using leaf extract of morinda pubescens. Asian J. Pharm. Clin. Res. 2012, 5, 159–162.
- Sri, S.K.; Prasad, T.N.V.K.V.; Panner, S.P.; Hussain, O.M. Synthesis, characterization and evaluation of effect of phytogenic zinc nanoparticles on soil exo-enzymes. Appl. Nanosci. 2014, 4, 819–827.
- Narayanan, K.B.; Sakthivel, N. Phytosynthesis of gold nanoparticles using leaf extract of Coleus amboinicus Lour. Mater. Charact. 2010, 61, 1232–1238.
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 1417–1424.
- Anu, K.; Singaravelu, G.; Murugan, K.; Benelli, G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): Biophysical characterization and cytotoxicity on vero cells. J. Clust. Sci. 2017, 28, 551–563.
- Jahanbin, R.; Yazdanshenas, P.; Rahimi, M.; Hajarizadeh, A.; Tvrda, E.; Nazari, S.A.; Mohammadi-Sangcheshmeh, A.; Ghanem, N. In vivo and in vitro evaluation of bull semen processed with zinc (zn) nanoparticles. Biol. Trace Elem. Res. 2021, 199, 126–135.
- Chen, J.; Wei, W.; ZhiSheng, W. Effect of nano-zinc oxide supplementation on rumen fermentation in vitro. Chin. J. Anim. Nutr. 2011, 23, 1415–1421.
- Mohamed, M.Y.; Ibrahim, E.M.M.; Abd El-Mola, A.M. Effect of selenium yeast and/or vitamin E supplemented rations on some physiological responses of post-lambing ossimi ewes under two different housing systems. Egypt. J. Nutr. Feeds 2017, 20, 361–378.
- Hosseini-Vardanjani, S.F.; Rezaei, J.; Karimi-Dehkordi, S.; Rouzbehan, Y. Effect of feeding nano-ZnO on performance, rumen fermentation, leukocytes, antioxidant capacity, blood serum enzymes and minerals of ewes. Small Rumin. Res. 2020, 191, 106170.
- Seven, P.T.; Seven, I.; Baykalir, B.G.; Mutlu, S.I.; Salem, A.Z.M. Nanotechnology and nano-propolis in animal production and health: An overview. Ital. J. Anim. Sci. 2018, 17, 921–930.
- Zhan, X.; Wang, M.; Zhao, R.; Li, W.; Xu, Z. Effects of different selenium source on selenium distribution, loin quality and antioxidant status in finishing pigs. Anim. Feed Sci. Technol. 2007, 132, 202–211.
- Adegbeye, M.J.; Elghandour, M.M.M.Y.; Barbabosa-Pliego, A.; Monroy, J.C.; Mellado, M.; Ravi, K.R.P.; Salem, A.Z.M. Nanoparticles in equine nutrition: Mechanism of action and application as feed additives. J. Equine Vet. Sci. 2019, 78, 29–37.
- Murthy, N. Investigation of Biochemical and Histopathological Modifiations Induced by Selenium Toxicity on Albino Mice; LAP Lambert Academic Publishing: Saarbrucken, Germany, 2013.
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4.
- Mohamed, A.H.; Mohamed, M.Y.; Ibrahim, K.; Abd El Ghany, F.T.F.; Mahgoup, A.A.S. Impact of nano-zinc oxide supplementation on productive performance and some biochemical parameters of ewes and offspring. Egypt. J. Sheep Goats Sci. 2017, 12, 1–16.
- Hashem, N.M.; Gonzalez-Bulnes, A. State-of-the-art and prospective of nanotechnologies for smart reproductive management of farm animals. Animals 2020, 10, 840.
- Rahman, H.U.; Qureshi, M.S.; Khan, R.U. Influence of dietary zinc on semen traits and seminal plasma antioxidant enzymes and trace minerals of beetal bucks. Reprod. Domest. Anim. 2014, 49, 1004–1007.
- Hosny, N.S.; Hashem, N.M.; Morsy, A.S.; Abo-Elezz, Z.R. Effects of organic selenium on the physiological response, blood metabolites, redox status, semen quality, and fertility of rabbit bucks kept under natural heat stress conditions. Front. Vet. Sci. 2020, 7, 290.
- Hernández-Sierra, J.F.; Ruiz, F.; Pena, D.C.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; Guillén, A.; Tapia-Pérez, H.; Castañón, G.M. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008, 4, 237–240.
- Abdel-Halim, B.R.; Moselhy, W.A.; Helmy, N.A. Developmental competence of bovine oocytes with increasing concentrations of nano-copper and nano-zinc particles during in vitro maturation. Asian Pac. J. Reprod. 2018, 7, 161–166.
- Abaspour, A.M.H.; Mamoei, M.; Tabatabaei, V.S.; Zareei, M.; Dadashpour, D.N. The effect of oral administration of zinc oxide nanoparticles on quantitative and qualitative properties of arabic ram sperm and some antioxidant parameters of seminal plasma in the non-breeding season. Arch. Razi. Inst. 2018, 73, 121–129.
- Lewis-Jones, D.I.; Aird, I.A.; Biljan, M.M.; Kingsland, C.R. Effects of sperm activity on zinc and fructose concentrations in seminal plasma. Hum. Reprod. 1996, 11, 2465–2467.
- Afifi, M.; Almaghrabi, O.A.; Kadasa, N.M. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. BioMed Res. Int. 2015, 2015, e153573.
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E. Impact of selenium nanoparticles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019, 126, 121–127.
- Konvicna, J.; Vargova, M.; Paulikova, I.; Kovac, G.; Kostecka, Z. Oxidative stress and antioxidant status in dairy cows during Cousins RJ, Liuzzi, J.P. Trace metal absorption and transport. prepartal and postpartal periods. Acta Vet. Brno 2015, 84, 133–140.
- Kachuee, R.; Abdi-Benemar, H.; Mansoori, Y.; Sánchez-Aparicio, P.; Seifdavati, J.; Elghandour, M.M.M.Y.; Guillén, R.J.; Salem, A.Z.M. Effects of sodium selenite, l-selenomethionine, and selenium nanoparticles during late pregnancy on selenium, zinc, copper, and iron concentrations in khalkhali goats and their kids. Biol. Trace. Elem. Res. 2019, 191, 389–402.
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Liu, Q.; Wang, Q.; Shi, L. Effect of elemental nano-selenium on feed digestibility, rumen fermentation, and purine derivatives in sheep. Anim. Feed Sci. Technol. 2011, 163, 136–142.
- Sadeghian, S.; Kojouri, G.A.; Mohebbi, A. Nanoparticles of selenium as species with stronger physiological effects in sheep in comparison with sodium selenite. Biol. Trace. Elem. Res. 2012, 146, 302–308.
- Wu, X.; Yao, J.; Yang, Z. Improved fetal hair follicle development by maternal supplement of selenium at nano size (Nano-Se). Livest. Sci. 2011, 142, 270–275.
- Xun, W.; Shi, L.; Yue, W.; Zhang, C.; Ren, Y.; Liu, Q. Effect of high-dose nano-selenium and selenium–yeast on feed digestibility, rumen fermentation, and purine derivatives in sheep. Biol. Trace Elem. Res. 2012, 150, 130–136.
- Kojouri, G.; Arbabi, F.; Mohebbi, A. The effects of selenium nanoparticles (SeNPs) on oxidant and antioxidant activities and neonatal lamb weight gain pattern. Comp. Clin. Pathol. 2020, 29, 369–374.
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52.
- Yaghmaie, P.; Ramin, A.; Asri-Rezaei, S.Z. Evaluation of glutathion peroxidase activity, trace minerals and weight gain following administration of selenium compounds in lambs. Vet. Res. Forum 2017, 8, 133–137.
- Zaboli, K.; Aliarabi, H.; Bahari, A.; Abbasalipourkabir, R. Role of dietary nano-zinc oxide on growth performance and blood levels of mineral: A study on Iranian Angora (Markhoz) goat kids. J. Pharm. Health Sci. 2013, 2, 19–26.
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial Properties of ZnO Nanomaterials: A Review. Ceram. Int. 2017, 43, 3940–3961.
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100.
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31.
- Shahin, M.A.; Khalil, W.A.; Saadeldin, I.M.; Swelum, A.A.; Mostafa, A.; El-Harairy, M.A. Comparison between the effects of adding vitamins, trace elements, and nanoparticles to shotor extender on the cryopreservation of dromedary camel epididymal spermatozoa. Animals 2020, 10, 78.
- Bąkowski, M.; Kiczorowska, B.; Samolińska, W.; Klebaniuk, R.; Lipiec, A. Silver and zinc nanoparticles in animal nutrition—A review. Ann. Anim. Sci. 2018, 18, 879–898.
- Amini, S.M.; Pirhajati, M.V. Selenium nanoparticles role in organ systems functionality and disorder. Nanomed. Res. J. 2018, 3, 117–124.
- Soumya, R.S.; Vineetha, V.P.; Raj, P.S.; Raghu, K.G. Beneficial properties of selenium incorporated guar gum nanoparticles against ischemia/reperfusion in cardiomyoblasts (H9c2). Metallomics 2014, 6, 2134–2147.
- Azarmi, F.; Kumar, P.; Mulheron, M. The exposure to coarse, fine and ultrafine particle emissions from concrete mixing, drilling and cutting activities. J. Hazard. Mater. 2014, 279, 268–279.
- Peralta-Videa, J.R.; Zhao, L.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15.
- Novoselec, J.; Šperanda, M.; Klir, Ž.; Mioč, B.; Steiner, Z.; Antunović, Z. Blood biochemical indicators and concentration of thyroid hormones in heavily pregnant and lactating ewes depending on selenium supplementation. Acta Vet. Brno 2017, 86, 353–363.
