Eight kaolinitic materials from the Lokoundje River at Kribi were sampled and investigated for their physical, chemical, mineralogical and thermal characteristics in order to evaluate their potential suitability as raw materials in ceramics. The Lokoundje kaolinitic materials are clayey to silty clayey and are predominantly composed of kaolinite and quartz. The alkali (Na2O + K2O) content ranges between 1 and 2.5 wt.%; these low values do not favor vitrification of the ceramics but may be improved through flux amendment. The presence of goethite in some samples limits their utilization in white ceramics. The minerals content, color, metallic sound, cohesion, linear shrinkage, flexural strength, bulk density, water absorption and microstructure were determined. The XRD data reveal that kaolinite and goethite were transformed, respectively, into mullite and hematite. The colors of the fired products are characteristic of their mineral assemblage. The metallic sound is indicative of low vitrification which is confirmed by the presence of cracks due to low flux contents. The cohesion is good to very good, due to the abundance of kaolinite. The physicomechanical properties increase with temperature as well as densification. The geochemical data show that the Lokoundje alluvial clays are suitable for the manufacture of white stoneware tiles.
- Kribi
- alluvial clays
- kaolinite
- valorization
- low mullitization
- white stoneware tiles
1. Introduction
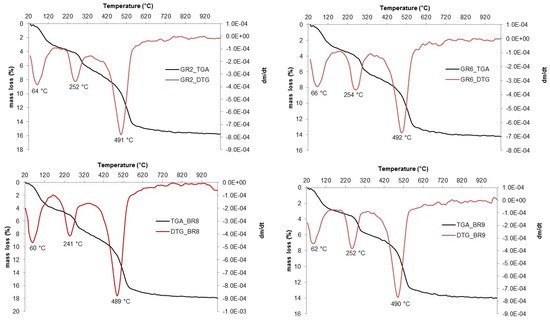
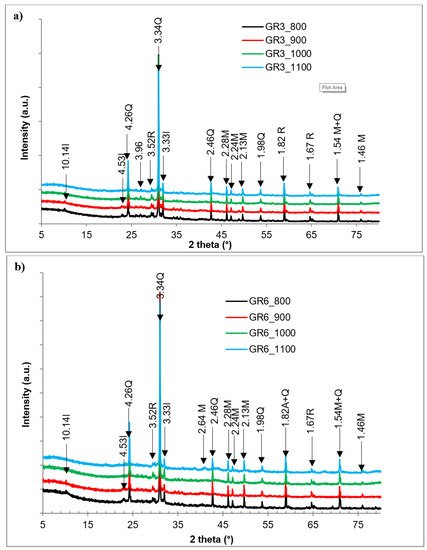
2. Thermal Behavior and XRD Mineralogical Evolution in Fired Samples

| Sample | Temperature (°C) | Color | Sound | Cohesion |
|---|---|---|---|---|
| BR-5 | 25 | Pale brown | - | - |
| 800 | Light red | Metallic | Good | |
| 900 | Light red | Metallic | Good | |
| 1000 | Light red | Metallic | Very good | |
| 1100 | Light red | Metallic | Very good | |
| BR-8 | 25 | Pale brown | - | - |
| 800 | Reddish yellow | Metallic | Good | |
| 900 | Reddish yellow | Metallic | Good | |
| 1000 | Light red | Metallic | Good | |
| 1100 | Light red | Metallic | Very good | |
| BR-9 | 25 | Light gray | - | - |
| 800 | Reddish yellow | Metallic | Weak | |
| 900 | Light red | Metallic | Average | |
| 1000 | Red | Metallic | Average | |
| 1100 | Dark red | Metallic | Good | |
| BR-11 | 25 | Reddish yellow | - | - |
| 800 | Reddish yellow | Metallic | Good | |
| 900 | Reddish yellow | Metallic | Good | |
| 1000 | Reddish yellow | Metallic | Very good | |
| 1100 | Light red | Metallic | Good | |
| GR-2 | 25 | Brownish gray | - | - |
| 800 | Reddish brown | Metallic | Good | |
| 900 | Pink | Metallic | Good | |
| 1000 | Pink | Metallic | Very good | |
| 1100 | Pink | Metallic | Good | |
| GR-3 | 25 | Light gray | - | - |
| 800 | Pinkish white | Metallic | Good | |
| 900 | Pinkish white | Metallic | Very good | |
| 1000 | Pinkish white | Metallic | Very good | |
| 1100 | White | Metallic | Good | |
| GR-6 | 25 | Brownish gray | - | - |
| 800 | Reddish yellow | Metallic | Good | |
| 900 | Reddish yellow | Metallic | Good | |
| 1000 | Pink | Metallic | Very good | |
| 1100 | Pink | Metallic | Very good | |
| GR-8 | 25 | Pale brown | - | - |
| 800 | Light red | Metallic | Good | |
| 900 | Light red | Metallic | Good | |
| 1000 | Light red | Metallic | Very good | |
| 1100 | Light red | Metallic | Very good |
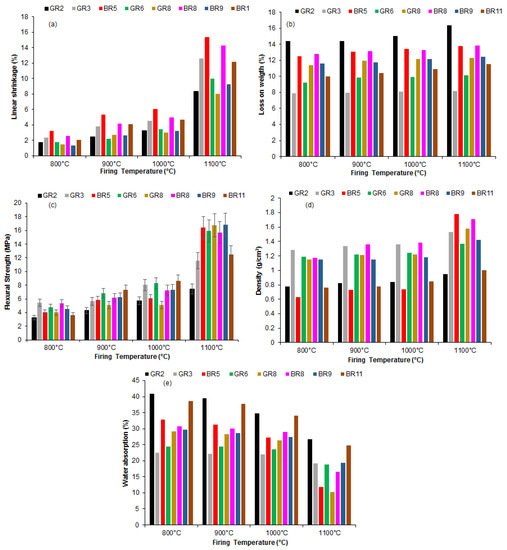
3. Technological Characterization of Fired Products
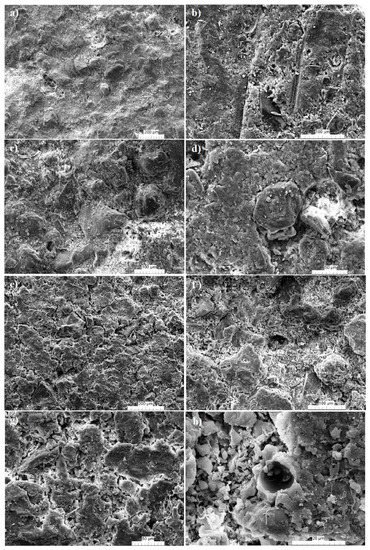
4. Microstructure
This entry is adapted from the peer-reviewed paper 10.3390/app11136118
References
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Clays in industry. In Developments in Clay Sciences. Bergaya, Theng, and Lagaly, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 13-978-0-08-044183-2.
- Anglisano, A.; Casas, L.; Anglisano, M.; Queralt, I. Application of Supervised Machine-Learning Methods for Attesting Provenance in Catalan Traditional Pottery Industry. Minerals 2020, 10, 8.
- Ekosse, E.G.I. Kaolin deposits and occurrences in Africa: Geology, mineralogy and utilization. Appl. Clay Sci. 2010, 50, 212–236.
- Khelifi, H.; Perrot, A.; Lecompte, T.; Ausias, G. Design of clay/cement mixtures for extruded building products. Mater. Struct. 2013, 46, 999–1010.
- Tchakouté, H.K.; Rüscher, C.; Hinsch, H.M.; Djobo, J.N.Y.; Kamseu, E.; Leonelli, C. Utilization of sodium waterglass from sugar cane bagasse ash as a new alternative hardener for producing metakaolin-based geopolymer cement. Geochemistry 2017, 77, 257–266.
- Cripps, H.R.; Isaias, N.P.; Jowet, A. The use of clays as an aid to water purification. Hydrometallurgy 1976, 1, 373–387.
- Beall, G.W. The use of organo-clays in water treatment. Appl. Clay Sci. 2003, 24, 11–20.
- Belibi Belibi, P.; Nguemtchouin, M.M.G.; Rivallin, M.; Ndi Nsami, J.; Sielechi, J.; Cerneaux, S.; Ngassoum, M.B.; Cretin, M. Microfiltration ceramic membranes from local Cameroonian clay applicable to water treatment. Ceram. Int. 2015, 41, 2752–2759.
- Djoufac, W.E.; Siéwé, J.M.; Njopwouo, D. A fixed-bed column for phosphate removal from aqueous solutions using an andosol-bagasse mixture. J. Environ. Manag. 2015, 151, 450–460.
- Siéwé, J.M.; Djoufac, W.E.; Djomgoue, P.; Njopwouo, D. Activation of clay surface of Bambouto’s andosol (Cameroon) with phosphate ions. Application for copper fixation in aqueous solution. Appl. Clay Sci. 2015, 114, 31–39.
- Zhang, G.; Lin, Y.; Wang, M. Remediation of copper polluted red soils with clay materials. J. Environ. Sci. 2011, 23, 461–467.
- Yuan, G.D.; Theng, B.K.G.; Churchman, G.J.; Gates, W.P. Clays and Clay Minerals for Pollution Control. Developments in Clay Science; Chapter 5.1; Elsevier: Amsterdam, The Netherlands, 2013; pp. 587–644.
- Tite, M.S. Ceramic production, provenance and use—A review. Archaeometry 2008, 50, 216–231.
- Fadil-Djenabou, S.; Ndjigui, P.-D.; Mbey, J.A. Morphological and physicochemical characterization of Ngaye alluvial clays (Northern Cameroon) and assessment of its suitability in ceramic production. J. Asian Ceram. Soc. 2015, 3, 50–58.
- Tsozue, D.; Nzeukou, N.A.; Mache, J.R.; Loweh, S.; Fagel, N. Mineralogical, physico-chemical and technological characterization of clays from Maroua (Far-North Cameroon) for use in ceramic bricks production. J. Build. Eng. 2017, 11, 17–24.
- Mbey, J.A.; Thomas, F.; Ngally Sabouang, C.J.; Liboum, N.D. An insight on the weakening of the interlayer bonds in a Cameroonian kaolinite through DMSO intercalation. Appl. Clay Sci. 2013, 83, 327–335.
- Mbey, J.A.; Hoppe, S.; Thomas, F. Cassava starch-kaolinite composite films. Thermal and mechanical properties related to filler-matrix interactions. Polym. Compos. 2015, 36, 184–191.
- Hu, P.; Yang, H. Insight into the physicochemical aspects of kaolin with different morphologies. Appl. Clay Sci. 2013, 74, 58–65.
- Mache, J.R.; Signing, P.; Njoya, A.; Kunyukubundo, F.; Mbey, J.A.; Njopwouo, D. Smectite clay from the Sabga deposit (Cameroon): Mineralogical and physicochemical properties. Clay Miner. 2013, 48, 499–512.
- Ndjigui, P.-D.; Mbey, J.A.; Nzeukou, N.A. Mineralogical, physical and mechanical features of ceramic products of the alluvial clastic clays from the Ngog-Lituba region, Southern Cameroon. J. Build. Eng. 2016, 5, 151–157.
- Nzeukou, N.A.; Fagel, N.; Njoya, A.; Kamgang Beyala, V.; Eko Medjo, R.; Melo Chinje, U. Mineralogy and physico-chemical properties of alluvial clays from Sanaga valley (Center, Cameroon): Suitability for ceramic application. Appl. Clay Sci. 2013, 83, 238–243.
- Ndjigui, P.-D.; Onana, V.L.; Sababa, E.; Bayiga, E.C. Mineralogy and geochemistry of the Lokoundje alluvial clays from the Kribi deposits, Cameroon Atlantic coast: Implications for their origin and depositional environment. J. Afri. Earth Sci. 2018, 143, 102–117.
- Ndjigui, P.-D.; Ebah Abeng, S.A.; Ekomane, E.; Nzeukou, N.A.; Ngo Mandeng, F.S.; Lindjeck, M.M. Mineralogy and geochemistry of pseudogley soils and recent alluvial clastic sediments in the Ngog-Lituba region, Southern Cameroon: An implication to their genesis. J. Afr. Earth Sci. 2015, 108, 1–14.
- Sonuparlak, B.; Sarikaya, M.; Aksay, I.A. Spinel phase formation during the 980°C exothermic reaction in the kaolinite-to-mullite reaction series. J. Am. Ceram. Soc. 1987, 70, 837–842.
- Carbajal, L.; Rubio-Marcos, F.; Bengochea, M.A.; Fernandez, J.F. Properties related phase evolution in porcelain ceramics. J. Eur. Ceram. Soc. 2007, 27, 4065–4069.
- Njoya, D.; Hajjaji, M.; Baçaoui, A.; Njopwouo, D. Microstructural characterization and influence of manufacturing parameters on technological properties of vitreous ceramic materials. Mater. Charact. 2010, 61, 289–295.
- Caspar, J.; McConville, C.J.; Lee, W.E. Microstructural development on firing illite and smectite clays compared with that in kaolinite. J. Am. Ceram. Soc. 2005, 88, 2267–2276.
- Mehta, S.N.; Sahu, K.P.; Tripathi, P.; Pyare, R.; Majhi, R.M. Influence of alumina and silica addition on the physico-mechanical and dielectric behavior of ceramic porcelain insulator at high sintering temperature. Bol. Soc. Española Cerámica Vidr. 2018, 57, 151–159.
- Chen, C.Y.; Lan, G.S.; Tan, W.H. Microstructural evolution of mullite during the sintering of kaolin powder compacts. Ceram. Int. 2006, 26, 715–720.
- Roudouane, H.T.; Mbey, J.A.; Bayiga, E.C.; Ndjigui, P.-D. Characterization and application tests of kaolinite clays from Aboudeia (southeastern Chad) in fired bricks making. Sci. Afr. 2020, 7, e00294.
- Behera, P.S.; Bhattacharyya, S. Effect of different alumina sources on phase formation and densification of single-phase mullite ceramic–Reference clay alumina system. Mater. Today Com. 2021, 26, 101818.
