Carbon dioxide (CO2) is a major greenhouse gas responsible for climate change. Diatoms, a natural sink of atmospheric CO2, can be cultivated industrially in autotrophic and mixotrophic modes for the purpose of CO2 sequestration. In addition, the metabolic diversity exhibited by this group of photosynthetic organisms provides avenues to redirect the captured carbon into products of value. These include lipids, omega-3 fatty acids, pigments, antioxidants, exopolysaccharides, sulphated polysaccharides, and other valuable metabolites that can be produced in environmentally sustainable bio-manufacturing processes. To realize the potential of diatoms, expansion of our knowledge of carbon supply, CO2 uptake and fixation by these organisms, in conjunction with ways to enhance metabolic routing of the fixed carbon to products of value is required.
- carbon supply
- CO2 uptake
- carbon fixation
- CCM
- biomanufacturing
- diatoms
1. Introduction—The Carbon Calamity
2. Diatoms for Bio-Based Manufacturing
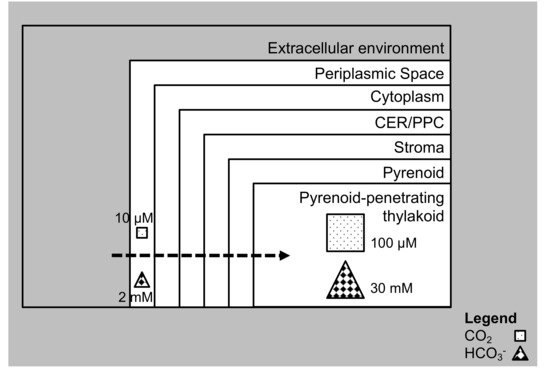
3. Carbon Assimilation in Diatoms
This entry is adapted from the peer-reviewed paper 10.3390/biology9080217
References
- Friedlingstein, P.; Jones, M.; O’Sullivan, M.; Andrew, R.; Hauck, J.; Peters, G.; Peters, W.; Pongratz, J.; Sitch, S.; Le Quéré, C.; et al. Global carbon budget 2019. Earth Syst. Sci. Data 2019, 11, 1783–1838.
- Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Jackson, R.B.; Korsbakken, J.I.; Le Quéré, C.; Peregon, A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies. Nat. Clim. Chang. 2020, 10, 3–6.
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.; Abernethy, S.; Andrew, R.M.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G.; et al. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Chang. 2020, 10, 1–7.
- Jackson, R.B.; Le Quéré, C.; Andrew, R.M.; Canadell, J.G.; Korsbakken, J.I.; Liu, Z.; Peters, G.P.; Zheng, B. Global energy growth is outpacing decarbonization. Environ. Res. Lett. 2018, 13, 12.
- Oelkers, E.H.; Cole, D.R. Carbon dioxide sequestration: A solution to a global problem. Elements 2008, 4, 305–310.
- Blomen, E.; Hendriks, C.; Neele, F. Capture technologies: Improvements and promising developments. Energy Procedia 2009, 1, 1505–1512.
- Luis, P. Use of Monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99.
- Han, K.; Ahn, C.K.; Lee, M.S.; Rhee, C.H.; Kim, J.Y.; Chun, H.D. Current status and challenges of the ammonia-based CO2 capture technologies toward commercialization. Int. J. Greenh. Gas Control 2013, 14, 270–281.
- Björn, L.O.; Govindjee. The evolution of photosynthesis and its environmental impact. In Photobiology: The Science of Light and Life, 3rd ed.; Springer: New York, NY, USA, 2015; pp. 207–230.
- Formighieri, C.; Franck, F.; Bassi, R. Regulation of the pigment optical density of an algal cell: Filling the gap between photosynthetic productivity in the laboratory and in mass culture. J. Biotechnol. 2012, 162, 115–123.
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280.
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; García, A.I.; Otero, M. Nutrients and pharmaceuticals removal from wastewater by culture and harvesting of Chlorella sorokiniana. Bioresour. Technol. 2015, 185, 276–284.
- Tsai, D.D.W.; Chen, P.H.; Ramaraj, R. The potential of carbon dioxide capture and sequestration with algae. Ecol. Eng. 2017, 98, 17–23.
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240.
- Medlin, L.K. Evolution of the diatoms: Major steps in their evolution and a review of the supporting molecular and morphological evidence. Phycologia 2016, 55, 79–103.
- Sorhannus, U. A nuclear-encoded small-subunit ribosomal RNA timescale for diatom evolution. Mar. Micropaleontol. 2007, 65, 1–12.
- Armbrust, E.V. The Life of Diatoms in the World’s Oceans. Nature 2009, 459, 185–192.
- Pančić, M.; Torres, R.R.; Almeda, R.; Kiørboe, T. Silicified cell walls as a defensive trait in diatoms. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190184.
- Taddei, L.; Stella, G.R.; Rogato, A.; Bailleul, B.; Fortunato, A.E.; Annunziata, R.; Sanges, R.; Thaler, M.; Lepetit, B.; Lavaud, J.; et al. Multisignal control of expression of the LHCX protein family in the marine diatom Phaeodactylum tricornutum. J. Exp. Bot. 2016, 67, 3939–3951.
- Walter, B.; Peters, J.; van Beusekom, J.E. The effect of constant darkness and short light periods on the survival and physiological fitness of two phytoplankton species and their growth potential after re-illumination. Aquat. Ecol. 2017, 51, 591–603.
- Bergkvist, J.; Klawonn, I.; Whitehouse, M.J.; Lavik, G.; Brüchert, V.; Ploug, H. Turbulence simultaneously stimulates small- and large-scale CO2 sequestration by chain-forming diatoms in the sea. Nat. Commun. 2018, 9, 1–10.
- Huisman, J.; Sharples, J.; Stroom, J.M.; Visser, P.M.; Kardinaal, W.E.A.; Verspagen, J.M.H.; Sommeijer, B. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 2004, 85, 2960–2970.
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A Diatom Cell Factory. Trends Biotechnol. 2020, 38, 606–622.
- Granum, E.; Raven, J.A.; Leegood, R.C. How do marine diatoms fix 10 billion tonnes of inorganic carbon per year? Can. J. Bot. 2005, 83, 898–908.
- Tréguer, P.J.; De La Rocha, C.L. The World Ocean Silica Cycle. Annu. Rev. Mar. Sci. 2013, 5, 477–501.
- Wilhelm, C.; Büchel, C.; Fisahn, J.; Goss, R.; Jakob, T.; LaRoche, J.; Lavaud, J.; Lohr, M.; Riebesell, U.; Stehfest, K.; et al. The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae. Protist 2006, 157, 91–124.
- Raven, J.A.; Waite, A.M. The evolution of silicification in diatoms: Inescapable sinking and sinking as escape? New Phytol. 2004, 162, 45–61.
- Jensen, E.L.; Yangüez, K.; Carrière, F.; Gontero, B. Storage compound accumulation in diatoms as response to elevated CO2 concentration. Biology 2020, 9, 5.
- Burkhardt, S.; Amoroso, G.; Riebesell, U.; Sültemeyer, D. CO2 and HCO3- uptake in marine diatoms acclimated to different CO2 concentrations. Limnol. Oceanogr. 2001, 46, 1378–1391.
- Buono, S.; Colucci, A.; Angelini, A.; Langellotti, A.L.; Massa, M.; Martello, A.; Fogliano, V.; Dibenedetto, A. Productivity and biochemical composition of Tetradesmus obliquus and Phaeodactylum tricornutum: Effects of different cultivation approaches. J. Appl. Phycol. 2016, 28, 3179–3192.
- Gérin, S.; Delhez, T.; Corato, A.; Remacle, C.; Franck, F. A novel culture medium for freshwater diatoms promotes efficient photoautotrophic batch production of biomass, fucoxanthin, and eicosapentaenoic acid. J. Appl. Phycol. 2020, 32, 1–16.
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-Green Algae with Chlorophyllide C 1,2. J. Phycol. 1972, 8, 10–14.
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60.
- Gagneux-Moreaux, S.; Moreau, C.; Gonzalez, J.L.; Cosson, R.P. Diatom Artificial Medium (DAM): A New Artificial Medium for the Diatom Haslea ostrearia and Other Marine Microalgae. J. Appl. Phycol. 2007, 19, 549–556.
- Goldman, J.C.; McCarthy, J.J. Steady state growth and ammonium uptake of a fast-growing marine diatom 1. Limnol. Oceanogr. 1978, 23, 695–703.
- Walne, P.R. Studies on the Food Value of Nineteen Genera of Algae to Juvenile Bivalves of the Genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish. Invest. Ser. 2 1970, 26.
- Hao, T.B.; Yang, Y.F.; Balamurugan, S.; Li, D.W.; Yang, W.D.; Li, H.Y. Enrichment of f/2 medium hyperaccumulates biomass and bioactive compounds in the diatom Phaeodactylum tricornutum. Algal Res. 2020, 47, 101872.
- Yang, R.; Wei, D. Improving fucoxanthin production in mixotrophic culture of marine diatom Phaeodactylum tricornutum by LED Light shift and nitrogen supplementation. Front. Bioeng. Biotechnol. 2020, 8, 820.
- Alonso, D.L.; Segura del Castillo, C.I.; Grima, E.M.; Cohen, Z. First insights into improvement of eicosapentaenoic acid content in Phaeodactylum tricornutum (bacillariophyceae) by induced mutagenesis 1. J. Phycol. 1996, 32, 339–345.
- Yi, Z.; Xu, M.; Magnusdottir, M.; Zhang, Y.; Brynjolfsson, S.; Fu, W.; Martin-Jézéquel, V. Photo-Oxidative Stress-Driven Mutagenesis and Adaptive Evolution on the marine diatom Phaeodactylum tricornutum for Enhanced Carotenoid Accumulation. Mar. Drugs 2015, 13, 6138–6151.
- Shifrin, N.S.; Chisholm, S.W. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J. Phycol. 1981, 17, 374–384.
- Lewin, J.C. The Taxonomic Position of Phaeodactylum tricornutum. J. Gen. Microbiol. 1958, 18, 427–432.
- Zhao, P.; Gu, W.; Wu, S.; Huang, A.; He, L.; Xie, X.; Gao, S.; Zhang, B.; Niu, J.; Peng Lin, A.; et al. Enhances the Growth of Phaeodactylum tricornutum Bohlin under Green Light and Low Temperature. Sci. Rep. 2014, 4, 3958.
- Poschenrieder, C.; Fernández, J.A.; Rubio, L.; Pérez, L.; Terés, J.; Barceló, J. Transport and Use of Bicarbonate in Plants: Current Knowledge and Challenges Ahead. Int. J. Mol. Sci. 2018, 19, 1352.
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The Boundless Carbon Cycle. Nat. Geosci. 2009, 2, 598–600.
- Hopkinson, B.M.; Dupont, C.L.; Matsuda, Y. The Physiology and Genetics of CO2 Concentrating Mechanisms in Model Diatoms. Curr. Opin. Plant Biol. 2016, 31, 51–57.
- Piiparinen, J.; Barth, D.; Eriksen, N.T.; Teir, S.; Spilling, K.; Wiebe, M.G. Microalgal CO2 capture at extreme PH values. Algal Res. 2018, 32, 321–328.
- Meiser, A.; Schmid-Staiger, U.; Trösch, W. Optimization of Eicosapentaenoic Acid Production by Phaeodactylum tricornutum in the Flat Panel Airlift (FPA) Reactor. J. Appl. Phycol. 2004, 16, 215–225.
- Negoro, M.; Shioji, N.; Miyamoto, K.; Micira, Y. Growth of microalgae in high CO2 gas and effects of SOX and NOX. Appl. Biochem. Biotechnol. 1991, 28–29, 877–886.
- Clement, R.; Jensen, E.; Prioretti, L.; Maberly, S.C.; Gontero, B. Diversity of CO2-Concentrating Mechanisms and Responses to CO2 Concentration in Marine and Freshwater Diatoms. J. Exp. Bot. 2017, 68, 3925–3935.
- Artamonova, E.Y.; Vasskog, T.; Eilertsen, H.C. Lipid Content and Fatty Acid Composition of Porosira Glacialis and Attheya Longicornis in Response to Carbon Dioxide (CO2) Aeration. PLoS ONE 2017, 12, e0177703.
- Baragi, L.V.; Khandeparker, L.; Anil, A.C. Influence of elevated temperature and pCO2 on the marine periphytic diatom Navicula distans and its associated organisms in culture. Hydrobiologia 2015, 762, 127–142.
- Boelen, P.; van de Poll, W.H.; van der Strate, H.J.; Neven, I.A.; Beardall, J.; Buma, A.G.J. Neither Elevated nor reduced CO2 affects the photophysiological performance of the marine antarctic diatom Chaetoceros brevis. J. Exp. Mar. Biol. Ecol. 2011, 406, 38–45.
- Fettke, J.; Fernie, A.R. Intracellular and Cell-to-Apoplast Compartmentation of Carbohydrate Metabolism. Trends Plant Sci. 2015, 20, 490–497.
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149.
- Badger, M.R.; Andrews, T.J.; Whitney, S.M.; Ludwig, M.; Yellowlees, D.C.; Leggat, W.; Price, G.D. The diversity and coevolution of Rubisco, Plastids, Pyrenoids, and Chloroplast-Based CO2-concentrating mechanisms in algae. Can. J. Bot. 1998, 76, 1052–1071.
- Kustka, A.B.; Milligan, A.J.; Zheng, H.; New, A.M.; Gates, C.; Bidle, K.D.; Reinfelder, J.R. Low CO2 results in a rearrangement of carbon metabolism to support C4 photosynthetic carbon assimilation in Thalassiosira pseudonana. New Phytol. 2014, 204, 507–520.
- Matthijs, M.; Fabris, M.; Obata, T.; Foubert, I.; Franco-Zorrilla, J.M.; Solano, R.; Fernie, A.R.; Vyverman, W.; Goossens, A. The Transcription Factor BZIP14 regulates the TCA Cycle in the diatom Phaeodactylum tricornutum. EMBO J. 2017, 36, 1559–1576.
