Overcoming the leukemia stem cell resistance to intensive chemotherapy has been an area of extensive research over the last two decades. Advances and greater understanding of the molecular biology of leukemia stem cells are in rapid progress. Targeted therapies are currently being used in clinical practice with reasonable response rates, but a cure is being achieved in only a small percentage of patients, most likely due to tumor mutational heterogeneity. A genetically engineered diphtheria toxin fused with interleukin-3 (SL-401 or tagraxofusp) has shown robust activity in blastic plasmacytoid dendritic cell neoplasm and promising response rates in different myeloid malignancies, including eradication of minimal residual disease. Multiple clinical trials are being conducted using this drug and the preliminary results are encouraging.
1. Introduction
The current treatment approach for myeloid malignancies is heavily dependent on intensive chemotherapy regimens that follow the old strategy of “one size fits all”. Novel targeted agents are emerging in clinical practice and clinical trials. Data on their efficacy and tolerability are promising, although no treatment has produced a cure thus far [
1]. Intensive chemotherapy treatments are designed to induce hypocellular marrow and allow hematopoietic stem cells to recover and resume normal production of blood cell components. The durability and duration of remission by this kind of therapy can vary and will depend primarily on the mutational signature of the disease and epigenetic aberration. In addition, the planned consolidation strategy, whether it is allogeneic stem cell transplantation or further consolidative chemotherapy, has an effect on leukemia-free survival and how deeply we can eradicate leukemic stem cells. New agents that target actionable mutations, such as FLT3 and IDH, have been approved for clinical practice [
2,
3]. However, they have not had a significant enough effect on overall survival rates to replace chemotherapy, partly because of the diversity of cytogenetic abnormalities and the clonal evolution of new mutations. Nevertheless, it is important to assess the performance status and comorbidities of these treatments before exploring which treatment approach is the most appropriate in terms of toxicity and survival. Unfortunately, even with the most intensive treatment, the risk of relapse is high in the first two years, especially in patients with adverse cytogenetic profiles [
4]. Extensive time and effort were spent in clinical trials optimizing the sequence of well-known acute leukemia chemotherapy protocols. There is a need to revolutionize the treatment of acute leukemia.
Unlike traditional chemotherapy, the specificity of the target on the tumor cells is the focus of the new era in cancer treatment. Various examples have been investigated in clinical trials, and many agents have been approved and are available in clinical practice. These include, for example, tyrosine kinase inhibitors to target the FLT3 mutation, monoclonal antibodies, bispecific antibodies, and chimeric antigen receptor T cell therapies. The ideal target, for example, in acute myeloid and lymphoid leukemia, is highly expressed in leukemic blasts, with minimal or no expression on normal hematopoietic cells, to induce deeper remission. Targets that have been studied in acute myeloid leukemia include CD33, CD135, FLT3, CXCR4, and vascular endothelial growth factor [
5]. This article focuses on the diphtheria toxin-interleukin 3 fusion protein as a targeted therapy to leukemic blasts.
Hematopoiesis is a complex process with multiple factors, undertaken to avoid the production of clones and leukemic stem cells. Interleukin 3 (IL-3) is one of the growth factors and cytokines that participate in this process at the level of granulocytic and monocytic lineage [
6]. Expression of the interleukin 3 receptor (IL-3R) starts at the CD34
+ hematopoietic cell and is maintained during all stages of development for granulocytes and monocyte precursors. Multiple investigators have targeted this receptor for the treatment of different types of myeloid leukemia [
7]. Most recently, experiments have used chimeric antigen receptor T cell technology to target CD123 in Hodgkin lymphoma in an effort to overcome the immunosuppressed microenvironment and allow the patient’s T lymphocytes to attack tumor cells [
8]. The structure of IL-3R consists of an α subunit, which is the site of ligand attachment and represents the specificity of the receptor, and a β subunit, which is shared with the granulocyte-macrophage colony-stimulating factor and is important for signal transduction, internalization of the ligand–receptor complexes, and activation through phosphorylation of the Ras pathway [
9].
The development of monoclonal antibodies and cytokines was a major advance in cancer treatment because of their ability to deliver cancer therapies, such as cytotoxic drugs, isotopes, and toxins. Toxins are powerful pro-apoptosis agents, and they achieve their cellular effect by various pathways. One of them is by shutting down protein synthesis [
10]. After removal of the natural binding domain of the toxins, they are conjugated to a monoclonal antibody or cytokine as their ligand [
11]. One toxin that has been used in cancer therapy is the diphtheria toxin, which is an exotoxin secreted by
Corynebacterium diphtheriae [
12]. This toxin inhibits protein synthesis in the host, which is the underlying pathophysiology of diphtheria infection [
8]. Previously, the diphtheria toxin was engineered to target interleukin-2 and was approved by the Food and Drug Administration (FDA) in 2002 for the treatment of cutaneous T cell lymphoma [
13]. However, because of the side effects, marketing of this drug was discontinued by the manufacturer.
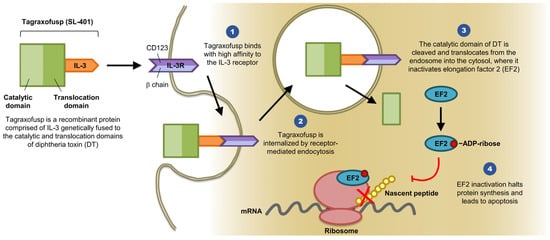
Diphtheria toxin consists of three domains and several linkers elements: The C terminus binding domain, the N terminus catalytic domain, and the translocation domain [
14]. IL-3 is a cytokine that promotes the differentiation of hematopoietic stem cells into various myeloid cells [
12]. IL-3R is composed of two subunits (α and β). The α subunit (CD123) directly binds to IL-3, whereas the β subunit (CDw131) functions as the signaling subunit (see
Figure 1) [
10]. The idea is to remove the diphtheria toxin binding domain and replace it with IL-3 to target leukemic stem cells that express CD123 and CDw131 by inhibiting intracellular protein synthesis and therefore inducing cell death.
Figure 1. SL-401 Structure and Mechanism of Action (provided by Stemline Therapeutics, Inc., New York, NY 10022, USA). Abbreviations: IL-3, interleukin 3; IL-3R, interleukin 3 receptor; DT, diphtheria toxin; EF2, elongation factor 2; mRNA, messenger ribonucleic acid; ADP, adenosine diphosphate.
2. Clinical Trials
The first in human trial was presented at the American Society of Clinical Oncology (ASCO) annual meeting in 2006. The preliminary findings showed manageable toxicity in elderly patients with acute myeloid leukemia (AML) or in those with relapsed or refractory disease [
18]. The study included 31 patients who received one cycle of SL-401. The findings showed mild toxicity and promising biologic activity. At the 2007 ASCO annual meeting, investigators reported a patient with AML who achieved complete remission (CR) for eight months as well as two cases of partial response (PR) [
19]. These patients were heavily pretreated, including bone marrow transplantation, and the cohort included patients with secondary AML either from a previous myelodysplastic syndrome or therapy-related AML. The major side effects reported in this abstract were mild to moderate and included transient fever, chills, hypotension, and hypoalbuminemia [
19].
Table 1 highlights the clinical trials for SL-401, adverse events, and response rates.
Table 1. Summary of SL-401 clinical trials in various hematologic neoplasms, baseline characteristics, adverse events and clinical outcomes (See page 14). Abbreviations: NCT, National Clinical Trial; R/R, Relapsed or refractory; AML, Acute myeloid leukemia; MDS, Myelodysplastic syndrome; CMML, Chronic myelomonocytic leukemia; BPDCN, Blastic plasmacytoid dendritic cell neoplasm; MM, Multiple myeloma; ORR, Overall response rate; CR, Complete response; PR, Partial response; LFT, Liver function tests; ASCO, American Society of Clinical Oncology; ASH, American Society of Hematology; CLS, Capillary leak syndrome; NA, Not available.
| SL-401 Clinical Trial Registry Numbers |
Primary Malignancy |
Status |
Study Start Date |
Phase |
Published Data |
Number of Patients |
Number of Cycles |
Age |
Male/Female |
Adverse Cytogenetics |
Intermediate Cytogenetics |
Relapsed (any) and Refractory |
Adverse Events |
ORR |
CR |
PR |
| NA |
R/R or elderly AML and high risk MDS |
Completed |
NA |
I |
Leukemia and Lymphoma 2007 |
45 |
1 |
67 (32–81) |
23/22 |
17 |
25 |
35 |
Grade III LFTs, Grade II fever, chills, low albumin and hypotension |
NA |
1 |
3 |
| NCT00397579 |
BPDCN |
Completed |
May 2013 |
I/II |
Blood 2014 for the BPDCN |
11 |
1 |
70 (40–77) |
11/0 |
NA |
NA |
7 |
Grade IV thrombocytopenia, Grade III LFTs and neutropenia |
NA |
5 |
0 |
| NA |
RR/AML and BPDCN |
NA |
NA |
NA |
ASH 2015 |
17 |
multiple |
63 |
NA |
NA |
NA |
NA |
Grade V CLS, Grade IV CLS, Grade III LFTs |
NA |
4 |
NA |
| NCT02270463 |
Consolidation Rx in adverse risk AML CR1 |
Recruiting |
February 2015 |
I/II |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
| NCT02268253 |
Advanced high risk MNP (SM, PED, MF, CMML) |
Recruiting |
December 2014 |
I/II |
ASH 2016 |
19 |
multiple |
69 (42–81) |
NA |
NA |
NA |
19 |
Grade III thrombocytopenia and anemia |
NA |
1 |
NA |
| NCT03113643 |
With AZA for Rx naïve AML/high risk MDS not eligible for standard Rx |
Recruiting |
June 2017 |
I |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
| NCT02661022 |
R/R MM |
Recruiting |
January 2016 |
I/II |
ASH 2016 |
2 |
multiple |
65 (63–67) |
NA |
NA |
NA |
2 |
Grade II thrombocytopenia and hypoalbuminemia |
NA |
0 |
2 |
| NCT02113982 |
BPDCN and R/R AML |
Recruiting |
September 2014 |
I/II |
ASCO 2016 (only BPDCN) |
18 |
multiple |
70 (45–82) |
NA |
NA |
NA |
10 |
CLS and thrombocytopenia |
87% |
8 |
NA |
Phase I data were first published in 2008 [
20] and highlighted the following points. First, the maximum tolerated dose is 12.5 μg/kg/day, which can be infused safely over 15 min, twice daily every 48 h with a total of six doses as one cycle. Second, toxicity and side effects were manageable, and no grade IV/V adverse events were reported. Grade II/III adverse events included transaminitis, fever, hypoalbuminemia, hypotension, and hypocalcemia. This cohort included elderly patients, most of whom had a disease that was refractory to standard therapy. The median age was 67 years, and only 11% of patients had previously untreated AML. In addition, 96% of patients had an intermediate cytogenetic profile. The trial reported one case of CR and two cases of PR (Both AML and MDS). All of the patients who showed some form of response had relapsed or refractory disease and the patients with previously untreated AML showed no response to the experimental treatment [
20].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines7010006