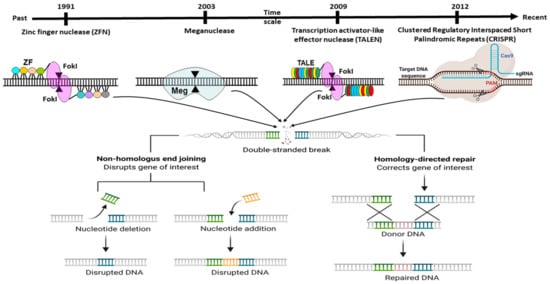
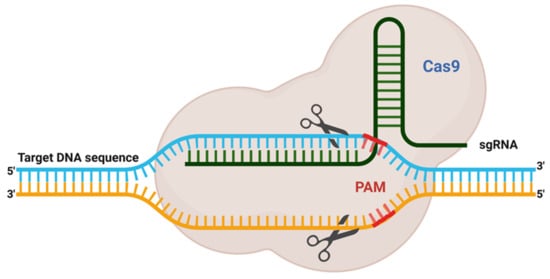
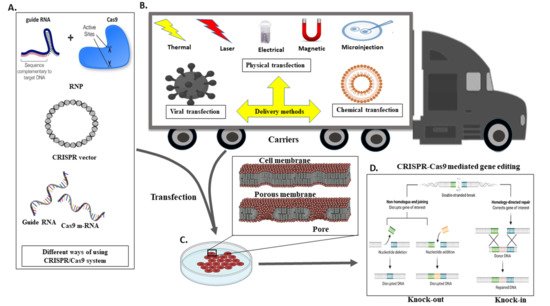
The delivery of Cas9 into cells is an imperative thought in gene editing. Adopted CRISPR–Cas can be utilised in different ways or formats, for instance, m-RNA (direct transfection of sgRNA and Cas9 RNA), DNA (vector-based strategy), and in the form of RNP (ribonuclease protein complex). For detailed information around the CRISPR-Cas transport systems, it would be ideal to follow the recent review article by Lino et al. [
68,
83] (
Figure 4A).
Figure 4. Different Methods of Delivering CRISPR/Cas9 into Cells. Schematic demonstration of in vivo CRISPR/Cas delivery modes and vehicles in numerous biological frameworks. Frameworks utilised to deliver CRISPR/Cas components can be separated into two major categories, CRISPR/Cas delivery mode and delivery carrier. (A) Three CRISPR/Cas delivery models, including protein (Cas protein with guide RNA as a ribonucleoprotein complex, RNP), DNA (plasmid encoding both the Cas protein and the gRNA), and RNA (mRNA for Cas protein translation and a separate gRNA), (B) Can be delivered into mammalians, aquacultures or plants by means of bacterial or viral vectors, chemical and physically directed delivery method, (C) To facilitate the delivery of the CRISPR system in the cell, transfection is accomplished by creating a membrane pore, and (D) Through the CRISPR framework, indel creation (knock out) or knock-in of a gene of interest in a targeted cell is possible.
The delivery method for CRISPR is very much similar to the standard transfection method for nucleic acid. CRISPR/Cas9 system delivery inside the cell usually conducts through either viral or chemical processes. Generally, physical processes are taken on electrical or mechanical forces to form transient pores in the membrane of cells, facilitating the update of CRISPR molecules. Moreover, recently, due to nanotechnology and microtechnologies, the physical method for transfection is in higher demand. For instance, nanostructure-mediated electroporation permits miniaturisation or shortened the physical transfection method to enhance transfection efficiency and precision [
68]. It has the advantage that it homogeneously treats cells with more minor or no viability damage to cells than bulk electroporation. Usually, CRISPR/Cas9 protein complexes have to be delivered in the cytoplasm of the transfected cells. To achieve efficient gene editing, CRISPR/Cas9 protein complexes must cross both the cell membrane and the nuclear membrane. As a result, the nuclear localisation sequence (NLS) directs the CRISPR/Cas9 system to the nucleus-encoded by the plasmid vector or the Cas9 protein. In the absence of an NLS sequence or signal, the CRISPR/Cas9 complex only enters the nucleus at the time of cell division when the membrane is disrupted [
68].
6. Genome Editing in Ruminants Such as Cattle and Buffalos
It has been anticipated that in the population of 7.6 billion humans globally, every ninth individual (821 million people) does not have sufficient food to cover an active life [
81]. Despite the lack of food, the human population is expected to rise to 8.5 billion in 2030, 9.7 billion in 2050, and 11.2 billion in 2100 [
82]. As a result, the United Nations’ Food and Agriculture Organization (FAO) predicts that total agricultural yield (crop yield and animal-based products) should rise to 60% to fulfil global demand. More specifically, this percentage is further contributed to by animal protein, such as meat production by 76%, and milk productivity will need to increase by 63%. In order to achieve this ultimatum goal, a precise and practical approach should be used [
84]. Meanwhile, genomics targets for genome engineering are possible by screening the differential expression using high throughput proteomics or genomics techniques [
85,
86,
87,
88]. In this regard, the generation of collective knowledge across the globe allows one to share and build the more efficient farm animals breeds [
89].
Genome-editing and transgenic innovations offer the chance for more significant gains over a shorter time. Until now, genome editing investigation in cattle has centred fundamentally on enhancing the efficiency of food productivity (e.g., meat and milk), animal health, and welfare (animal population, surveyed or hornlessness and disease), generate all-male offspring, eradication of allergens from products (e.g., beta-lactoglobulin knock-out). On the other hand, genome editing might be utilised to precisely knock-in valuable alleles (such as heat tolerance, illness resistance), as well as haplotypes into our native locally well-adapted cattle breeds genome, subsequently to improve their productivity [
90]. We recently used the buffalo mammary epithelial cells to understand lactogenic signalling [
91,
92].
Early research was majorly focused on animal growth. Skeletal muscle gives meat for human utilisation or consumption, consisting of muscle fibres, intramuscular adipose tissues, and connective tissues [
93]. The importance of growth hormone (GH) and insulin-like growth factor I (IGF-I) in regulating body size in developing animals has long been recognised. GH and IGF-I play an essential role in muscle growth, both before and after birth [
94]. The GH–IGF axis (growth hormone-insulin-like growth factor axis), regulated by the pituitary gland and liver, is responsible for muscle growth and body mass [
76]. GH, on the other hand, induces the development of IGF-I in almost all tissues. The liver is the only organ that can primarily produce serum IGF-I. The pituitary gland produces GH, which stimulates the development of IGF-I in other tissues (liver and muscle). Even though some cIGF-I is released from other tissues, such as muscle, the liver is the most common source of circulating IGF-I (cIGF-I). cIGF-I is a component of the negative feedback loop that controls GH secretion [
94]. IGF-1 derived from both muscle and liver plays a crucial role in myogenesis [
95]. At the same time, a mutation in the IGF-2 gene’s regulatory function has been linked to increased muscle growth in pigs [
96]. In recent years, effective microinjection of the GH and IGF-1 genes into pig zygotes has been reported. Later, two lines of GH-expressing transgene pigs gained 11.1 and 13.7 percent more mass than control pigs [
97,
98,
99]. When transgenic technology is combined with recent genome editing technology, it creates a new age or property for animal protein that could affect animal welfare, while meeting human diet demands. The cloned pig, for example, that expresses the fat-1 gene from the nematode C. elegans, has a lower ratio of n–6 to n–3 fatty acids. A higher ratio of n–6 to n–3 fatty acids has been linked to poor bone health in humans. A lower ratio is related to healthier bone properties; thus, reducing both fatty acids can have nutritional health benefits in a diet [
100].
Furthermore, related modifications have been observed in pigs containing the C. elegans n–3 fatty acid desaturase gene (encoded by the fat-1 gene) [
101,
102,
103]. Similar findings were obtained when CRISPR/Cas9 was used to insert the fat-1 gene into the pig in the rosa 26 locus [
104]. This is in proximity with gene alteration (genetic manipulation), which depends on the internalisation of the artificial gene (transgenes) to improve characteristic traits in animals. The genome/gene editing method allows us to make precise and error-free modifications to a livestock animal’s genome, to increase productivity, production, and infection resistance. In the genome editing region, targeted gene editing of the myostatin gene is a popular goal for increasing growth and muscle production. They were first noticed in heavily muscled sheep and cattle like Piedmontese and Belgian Blue cattle and the Texel sheep breed. Additionally, it was discovered that decreased expression of the myostatin gene (also known as GDF8, or growth differentiation factor 8) results in increased muscle growth. Single-nucleotide polymorphisms in the myostatin gene trigger a fundamental genetic change. The Piedmontese and Belgian Blue have a single-nucleotide polymorphism in the myostatin gene and an 11-bp deletion in the myostatin gene [
104,
105,
106].