Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Food Science & Technology
Liposomes are small spherical particles composed mainly from different kind of lipids, which are organised in the form of one or more lipid bilayers.
- model lipid bilayers
- bioactive compounds
- membrane fluidity
- membrane kinetics
- essential methods for liposome/vesicle preparation
1. Introduction
Liposomes are small spherical particles composed mainly from different kind of lipids, which are organised in the form of one or more lipid bilayers. This organisation allows them to be used as a simple approximation to living cells. As the liposomes are fairly simple to produce, relatively stable and also much less delicate to handle than the human cell lines, for example, they can be a very useful tool for determining the basic interactions of bioactive compounds or extracts with the lipid bilayers. Therefore, many medicinal and food-based studies are performed on them [1].
In food industry, one of the most widely used bioactive compounds are plant polyphenols, since they are numerous and quite easy to obtain, even with as simple procedure as ethanol extraction. They are one of the most widely distributed and complex groups of compounds in the plant kingdom, with >8000 phenolic structures currently known. Many of these have strong biological activities, mainly strong antioxidant capacity (free radical scavenging and metal chelation activity). While some polyphenols also showed possible beneficial implications in medicine, e.g., in treatment and prevention of cancer, cardiovascular disease and other pathologies, other showed considerable antimicrobial activity. The most important representatives of such phenols are phenolic acids, hydroxycinnamic acids and flavonoids, while certain individual members of other groups of polyphenols are important as well. Polyphenols are an integral part of the human diet, and they have gained more and more attention because of their potential beneficial effects on human health [2]. However, to understand their full potential, extensive investigations into their interactions with lipid membranes, among other things, have to be carried out.
As the polyphenols have a very diverse and complex structures [2], each molecule has to be studied individually in order to determine its solubility and positioning on or within the lipid membrane [3][4], its permeability through the bilayer [5], and its interactions with the membranes under different conditions [6]. Sometimes, synergistic effects among specific polyphenols and/or other compounds are also studied, in order to better understand the interactions of complex extracts, such as propolis, with the membranes [7]. Since liposomes represent simplified versions of the cells, they are a very suitable solution for studying the effects polyphenols might have on the lipid bilayers.
Indeed, many methods are applied to study how different compounds interact with lipid bilayers, and in this review we focus on the most basic ones: differential scanning calorimetry (DSC), isothermal titration calorimetry (ITC), electron paramagnetic resonance (EPR) spectrometry, nuclear magnetic resonance (NMR) spectrometry, fluorescence anisotropy/polarisation spectroscopy, thiobarbituric acid reactive substance (TBARS) assays, calcein release assays, 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene, boron dipyrromethene (BODIPY 581/591 C11) assays, confocal microscopy, and different methods for determining sizes and surface charges of liposomes.
2. Preparation of Liposomes
In order to study the membrane-polyphenol interactions, the lipid membranes have to be obtained/prepared first. The options are many [8]. More complex methods include using the membranes of the human cancer cell lines [9] or obtaining them from living cells, such as erythrocyte ghosts [10][11]. Others include creating them as tethered [12], planar [13] or other none-spherical lipid bilayers [14], while the simplest methods are based on preparing them as simple liposomes [15]. The first artificially made liposomes were created in the 40’s by J.Y. Johnson, whom patented the method for the use in pharmaceutical industry (I. G. Farbenindustrie Aktiengesellschaft). The so called “depots” were obtained when different fats or fatty oils were mixed with various water solutions. A few decades later in the 60’s, unaware of this patent, similar method for creating liposomes were discovered by different researchers, and with the extensive work by Alec Douglas Bangham, one of the first to discovered how to obtained them and also how to use them as a membrane model systems, liposomes quickly gained in popularity [16]. They were soon recognised as robust, simply-created and efficient replacements for model membrane systems and are in use ever since. As the preparation of liposomes is one of the easiest, most cost-affordable options, many studies are based on them.
There are many different types of liposomes, some of which are more suitable for specific research techniques than others. The most basic types are small unilamellar vesicles (SUVs), large unilamellar vesicles (LUVs), giant unilamellar vesicles (GUVs), multilamellar vesicles (MLVs), and multivesicular vesicles (MVVs) [17], as illustrated in Figure 1. SUVs, LUVs and MLVs are used the most, especially for DSC (i.e., MLVs), polarisation and anisotropy or for EPR spectrometry measurements (i.e., LUVs and SUVs) [18]. To obtain MLVs, the thin-film or proliposomal methods are usually applied, although other methods can be used as well.
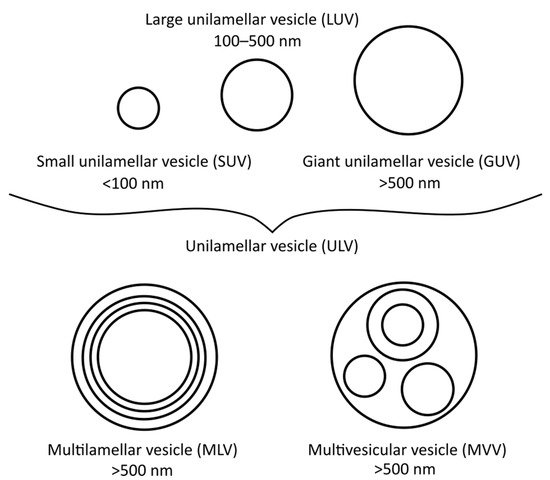
Figure 1. Different vesicle/liposome types and their diameters.
2.1. Thin-Film Method
The thin-film method is one of the most widely used liposome preparation techniques. It is based on the creation of a thin film of lipids, which is formed on the inner wall of the rotary evaporator flask. The film thus obtained is latter hydrated with a water or buffer solution. Before the hydration, it is very important that the lipid film, as well as the water/buffer solution, are preheated above the lipids transitional temperature (Tm), when necessary, in order to enable a smoother creation of the bilayer. This, coupled with the vigorous shaking and potential sonication in ultrasonic bath, enables the film to peel of the flask and form liposomes. The liposomes prepared in this way are MLVs of different sizes [19]. The encapsulating substance can be added with the lipids before the formation of the thin film (in the case of lipophilic compounds) or with the water/buffer solution (in the case of hydrophilic compounds). The biggest plus of this method is its high reproducibility even when working with small quantities of compounds, while its biggest minus is its low encapsulation efficiency. It is useful especially for encapsulation of lipophilic components in small, pharmaceutical quantities.
The method has been used by many different researchers [18][20][21][22][23], and the one described here was adapted from Lasch, Weissig [24] and Lasic [25]:
At first, 2–20 mg of lipids (e.g., 1,2-dipalmitoyl-sn-glycero-3-phosphocholine [DPPC], or 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine [POPC]) are weighted in a pre-weighted 10 mL rotary flask, then dissolved in 2–4 mL methanol:chloroform mixture (3:7, v/v). Solvent is then evaporated at 200–300 mbar (rotary vacuum evaporator) during heating in a water bath at 35–45 °C at appropriate rotation speed. The thin film is formed. It is further dried at high vacuum (5–10 mbar) until constant weight (4 h or overnight (small volumes (<1 mL) can be dried by purging with dry nitrogen)). Before the formation of the liposomes (hydration), the thin film and water/buffer are preheated above Tm of the chosen lipids. This is especially important in the case of lipids with high Tm (e.g., DPPC), whereas in the case of lipids with Tm around room temperature or lower (e.g., POPC) this is not necessary. The water/buffer is then added to yield concentrations between 0.5–10 mg/mL of liposomes. The lipids are hydrated at temperatures above Tm in closed rotary flask (45 min; occasional shaking and sonication in the ultrasonic bath (≈30 s/sonication); addition of small round glass beads if necessary). Finally, the formed MLVs are sonicated in ultrasonic bath for 3 min, stored in plastic microcentrifuge tubes, aerated with nitrogen gas, frozen in liquid nitrogen and stored at −80 °C.
Liposomes stored in this manner are stable for a long period of time. For the preparation of the liposomes with added compounds (e.g., polyphenols), the compounds can be added to the lipids before the preparation of the thin film (i.e., dissolved in the chloroform/methanol mixture, for lipophilic compounds), or before the formation of the liposomes (i.e., dissolved in the hydration solution, for hydrophilic compounds).
Other types of liposomes (e.g., SUVs and LUVs) can be prepared from these MLVs by either sonication with a high-intensity ultrasonic cell disruptor (for SUV preparation) or using the extrusion method (for SUV and LUV preparation). For sonication, MLVs are kept in an ice-cold bath during the sonication (e.g., 15 min, 10 s on/off intervals, 40% amplitude, 750 W, for 1 mL MLVs at 1 mg/mL). The SUVs obtained are centrifuged at 15,800× g (RCF) for 6 min and then used in the analysis [18]. For the extrusion method (Figure 2), the MLVs need to be extruded multiple times through a thin polycarbonate membrane filter of the required pore size using an extruder, thus creating different sizes of liposomes depending on the filter pore size [21].
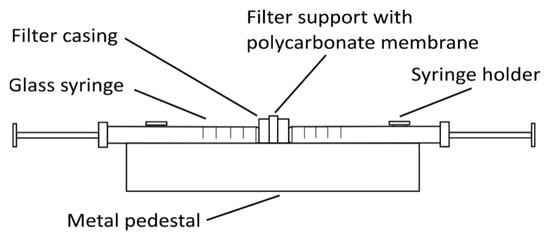
Figure 2. Simple glass syringe hand extruder. The polycarbonate membrane filter of the selected pore size is inserted into the filter support frame. One syringe is then filled with the liposome solution, which is extruded multiple-times from one syringe to the other through the membrane filter, to create small or large unilamellar vesicles.
2.2. Proliposome Method
The proliposomal method might be the simplest method for obtaining the liposomes. In contrast to thin-film method, its biggest minus is its relatively poor reproducibility when preparing smaller quantities of liposomes, while it yields much higher encapsulation efficiencies. The method is based on dissolving the lipids in water and ethanol, while stirring at 60 °C for roughly 10 min, to create a smooth lipid paste. After that, the lipids are cooled down and water/buffer is added in drops, while stirring. The suspension is then hydrated for 1 h as MLVs are formed. The method is very useful for preparation of larger quantities of liposomes, especially for assays like TBARS.
The method was used by different researchers [26][27][28], and the one described here was adapted from Perrett, Golding [29]. In brief:
Between 0.1–1 g of lipids are weighted in a small glass beaker, then 96% ethanol and water/buffer (final lipid:ethanol:buffer ratio of 1:1:2 (w/w/w)) are added. The mixture is heated at 60 °C in a water bath for ≈10 min with stirring on a magnetic stirrer at 600–800 rpm until a fine paste is obtained. The lipid paste is cooled to room temperature and an appropriate amount of water/buffer at room temperature is added in small drops during stirring. The MLVs are formed. Those MLVs are further hydrated during stirring for 1 h at room temperature, following by sonication of liposomes for 3 min in sonication bath. The obtained liposomes are stored for up to 2 days in the fridge (4 °C), or are transferred to centrifuge tubes, aerated with nitrogen gas, frozen in liquid nitrogen and stored at −80 °C.
To obtain proliposomes with encapsulated compounds, the compounds can be added with the ethanol before (for lipophilic compounds), or with the water/buffer during the formation of the liposomes (for hydrophilic compounds).
2.3. Injection Methods
There are many variations to this method, although the injection of the lipid suspension (for either hydrophobic or hydrophilic organic solvents) into the water phase is characteristic of all of them. In general, the main advantages here are the simplicity of the preparation procedures, and the preparation of large quantities of liposomes, although this also requires large amounts of usually expensive compounds [25][30]. In this review we will briefly discus the ethanol and ether injection methods.
2.3.1. Ethanol Injection
Ethanol injection method is used for preparing liposomes ranging between 30–170 nm, and is usually used to prepare SUVs. The size itself depends on the concentration of lipids and the injection speed. When preparing liposomes using this method, the lipids dissolved in an organic solvent (in this case ethanol) are injected into the water phase during stirring, and then the solvent is removed. The solution is then left hydrating during stirring for another 15 min. The ethanol can be removed from the liposome suspension either by rotary evaporation or by centrifugation through a silica gel column.
The limitations of this method are very poor encapsulation efficiency of hydrophilic compounds, the relatively limited solubility of lipids in ethanol, and the limited concentrations of lipids in the final solution due to the high ethanol content in it. Also, ethanol concentration should not exceed 7.5%, in order to prevent liposome destabilisation, which impacts the amount of the lipids that can be added [25][31][32]. The method is otherwise quite useful for preparation of large quantities of liposomes on industrial scale [33].
2.3.2. Ether Injection
Ether injection method is very similar to the ethanol injection method, with the important exception, that the lipid solvent used in this case, ether, does not mix with water at all. This, coupled with higher lipids solubility in ether opposed to ethanol and the fact that ether does not disrupt liposome formation, enables the preparation of higher concentrations of liposomes. After injection, ether is removed from the solution in the same manner as ethanol.
There are also some downsides to this method, as ether and water phases need to be at different temperatures during the injection procedure, ether might affect the encapsulation of some compounds, and the liposomes formed have very heterogeneous shapes. Injecting the lipid suspension into the water/buffer should also be slower than for ethanol injection method, and it is advised to do this under vacuum. However, the liposomes produced using this method have higher encapsulation efficiencies. In contrast to ethanol injection method, LUVs are formed rather than SUVs [25][30]. A fine example of this method was applied by [34].
2.4. Emulsification Method
The emulsification method is similar to the injection methods, as the lipids are again dissolved in organic solvent and then both organic and water phases are mixed together. In contrast to the injection method, however, smaller quantities of water phase are added to the organic phase (i.e., at an inverted organic/water phase ratio), and not the other way around. Also, the organic phase is not removed soon afterwards—instead, an emulsion of water in organic phase is formed. The lipids form a monolayer around water droplets, and then the organic solvent is evaporated. With the evaporation of the organic solvent, liposomes are formed around water particles. The mayor plus of emulsification method is, that it provides higher encapsulation efficiencies compared to the injection methods. Among many different versions of emulsification method known, the reverse-phase evaporation is one of the most basic ones [25][30].
The reverse-phase evaporation method was used by different researchers [35][36], and the one described here was applied by Cortesi, Esposito [37]. Briefly:
First, 40 mg of phosphatidylcholine is dissolved in 1 mL chloroform/methanol (2:1, v/v) in a 25-mL round-bottomed flask, followed by evaporation of solvent by rotary evaporation. Then either a different (i.e., ethanol or diethyl ether) or the same organic solvent is added, to yield a final volume of 8 mL. Further, additional 2 mL of a water/buffer solution is added and organic phase is evaporated by rotary evaporation at 40 °C under reduced pressure. The acquired aqueous suspension is sonicated in ultrasonic bath, during additional shaking. The liposomes obtained in this way are mainly LUVs, with a mean diameter of around 320–480 nm. To prepare smaller liposomes, these liposomes can be sonicated or extruded, as indicated before. For encapsulation of compounds, lipophilic molecules are dissolved in the organic phase together with the lipids, while the hydrophilic molecules are added together with the water phase.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22126547
References
- Lasic, D.D.; Papahadjopoulos, D. Medical Applications of Liposomes; Elsevier Science Publishers B. V.: Amsterdam, The Netherlands, 1998; p. 779.
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333.
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J.-M.; Ghoul, M. Solubility of Flavonoids in Organic Solvents. J. Chem. Eng. Data 2007, 52, 1552–1556.
- Scheidt, H.A.; Pampel, A.; Nissler, L.; Gebhardt, R.; Huster, D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. BBA Biomembr. 2004, 1663, 97–107.
- Tammela, P.; Laitinen, L.; Galkin, A.; Wennberg, T.; Heczko, R.; Vuorela, H.; Slotte, J.P.; Vuorela, P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch. Biochem. Biophys. 2004, 425, 193–199.
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40.
- Amoros, M.; Simões, C.M.; Girre, L.; Sauvager, F.; Cormier, M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992, 55, 1732–1740.
- Siontorou, C.G.; Nikoleli, G.-P.; Nikolelis, D.P.; Karapetis, S.K. Artificial Lipid Membranes: Past, Present, and Future. Membranes 2017, 7, 38.
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin—Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936.
- Hoffman, J.F. Physiological characteristics of human red blood cell ghosts. J. Gen. Physiol. 1958, 42, 9–28.
- Simons, T.J. The preparation of human red cell ghosts containing calcium buffers. J. Physiol. 1976, 256, 209–225.
- Giess, F.; Friedrich, M.G.; Heberle, J.; Naumann, R.L.; Knoll, W. The protein-tethered lipid bilayer: A novel mimic of the biological membrane. Biophys. J. 2004, 87, 3213–3220.
- Movileanu, L.; Neagoe, I.; Flonta, M.L. Interaction of the antioxidant flavonoid quercetin with planar lipid bilayers. Int. J. Pharmaceut. 2000, 205, 135–146.
- Henn, F.A.; Thompson, T.E. Synthetic Lipid Bilayer Membranes. Annu. Rev. Biochem. 1969, 38, 241–262.
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharmaceut. 2009, 6, 1264–1276.
- Bangham, A.D.; Hill, M.W.; Miller, N.G.A. Preparation and use of liposomes as models of biological membranes. In Methods in Membrane Biology; Korn, E.D., Ed.; Springer: Boston, MA, USA, 1974; Volume 1, pp. 1–68.
- Dua, J.S.; Rana, P.A.; Bhandari, D.K. Liposome: Methods of preparation and applications. Int. J. Pharm. Stud. Res. 2012, III, 14–20.
- Abram, V.; Berlec, B.; Ota, A.; Šentjurc, M.; Blatnik, P.; Ulrih, N.P. Effect of flavonoid structure on the fluidity of model lipid membranes. Food Chem. 2013, 139, 804–813.
- Weissig, V. Liposomes: Methods and Protocols: Pharmaceutical Nanocarriers; Humana Press (Springer Science+Business Media): New York, NY, USA, 2010; Volume 1, p. 564.
- Elhissi, A.M.; O’Neill, M.A.; Roberts, S.A.; Taylor, K.M. A calorimetric study of dimyristoylphosphatidylcholine phase transitions and steroid-liposome interactions for liposomes prepared by thin film and proliposome methods. Int. J. Pharmaceut. 2006, 320, 124–130.
- Isailović, B.D.; Kostić, I.T.; Zvonar, A.; Đorđević, V.B.; Gašperlin, M.; Nedović, V.A.; Bugarski, B.M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. 2013, 19, 181–189.
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative Effects of Cholesterol and β-Sitosterol on the Liposome Membrane Characteristics. Eur. J. Lipid Sci. Tech. 2018, 120, 1800039.
- Ota, A.; Abramovič, H.; Abram, V.; Poklar Ulrih, N. Interactions of p-coumaric, caffeic and ferulic acids and their styrenes with model lipid membranes. Food Chem. 2011, 125, 1256–1261.
- Lasch, J.; Weissig, V.; Brandl, M. Preparation of liposomes. In Liposomes—A Practical Approach, 2nd ed.; Torchilin, V.P., Weissig, V., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 3–30.
- Lasic, D.D. Liposomes, from Physics to Application; Elsevier Science Publishers B. V.: Amsterdam, The Netherlands, 1993; p. 575.
- Ishikawa, H.; Shimoda, Y.; Matsumoto, K. Preparation of liposomal microcapsules by proliposome method with soybean lecithin. J. Fac. Agric. 2004, 49, 119–127.
- Elhissi, A.; Gill, H.; Ahmed, W.; Taylor, K. Vibrating-mesh nebulization of liposomes generated using an ethanol-based proliposome technology. J. Lipos. Res. 2011, 21, 173–180.
- Istenič, K.; Cerc Korošec, R.; Poklar Ulrih, N. Encapsulation of (-)-epigallocatechin gallate into liposomes and into alginate or chitosan microparticles reinforced with liposomes. J. Sci. Food Agric. 2016, 96, 4623–4632.
- Perrett, S.; Golding, M.; Williams, W.P. A simple method for the preparation of liposomes for pharmaceutical applications: Characterization of the liposomes. J. Pharm. Pharmacol. 1991, 43, 154–161.
- Torchilin, V.P.; Weissig, V. Liposomes—A Practical Approach, 2nd ed.; Oxford University Press: Oxford, UK, 2003; p. 369.
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Lipos. Res. 2010, 20, 228–243.
- Pons, M.; Foradada, M.; Estelrich, J. Liposomes obtained by the ethanol injection method. Int. J. Pharmaceut. 1993, 95, 51–56.
- Charcosset, C.; Juban, A.; Valour, J.-P.; Urbaniak, S.; Fessi, H. Preparation of liposomes at large scale using the ethanol injection method: Effect of scale-up and injection devices. Chem. Eng. Res. Des. 2015, 94, 508–515.
- Pham, H.L.; Shaw, P.N.; Davies, N.M. Preparation of immuno-stimulating complexes (ISCOMs) by ether injection. Int. J. Pharmaceut. 2006, 310, 196–202.
- Chen, G.; Li, D.; Jin, Y.; Zhang, W.; Teng, L.; Bunt, C.; Wen, J. Deformable liposomes by reverse-phase evaporation method for an enhanced skin delivery of (+)-catechin. Drug Dev. Ind. Pharm. 2014, 40, 260–265.
- Rojanapanthu, P.; Sarisuta, N.; Chaturon, K.; Kraisintu, K. Physicochemical properties of amphotericin B liposomes prepared by reverse-phase evaporation method. Drug Dev. Ind. Pharm. 2003, 29, 31–37.
- Cortesi, R.; Esposito, E.; Gambarin, S.; Telloli, P.; Menegatti, E.; Nastruzzi, C. Preparation of liposomes by reverse-phase evaporation using alternative organic solvents. J. Microencapsul. 1999, 16, 251–256.
This entry is offline, you can click here to edit this entry!
