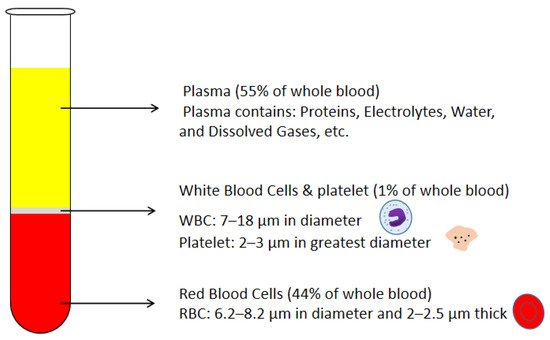
Blood plasma is the most commonly used biofluid in disease diagnostic and biomedical analysis due to it contains various biomarkers. The majority of the blood plasma separation is still handled with centrifugation, which is off-chip and time-consuming. Therefore, in the Lab-on-a-chip (LOC) field, an effective microfluidic blood plasma separation platform attracts researchers’ attention globally. Blood plasma self-separation technologies are usually divided into two categories: active self-separation and passive self-separation. Passive self-separation technologies, in contrast with active self-separation, only rely on microchannel geometry, microfluidic phenomena and hydrodynamic forces. Passive self-separation devices are driven by the capillary flow, which is generated due to the characteristics of the surface of the channel and its interaction with the fluid. Comparing to the active plasma separation techniques, passive plasma separation methods are more considered in the microfluidic platform, owing to their ease of fabrication, portable, user-friendly features.
- passive self-separation
- microfluidics
- microfiltration
- sedimentation
- Dean vortex
- hydrophilicity
1. Introduction
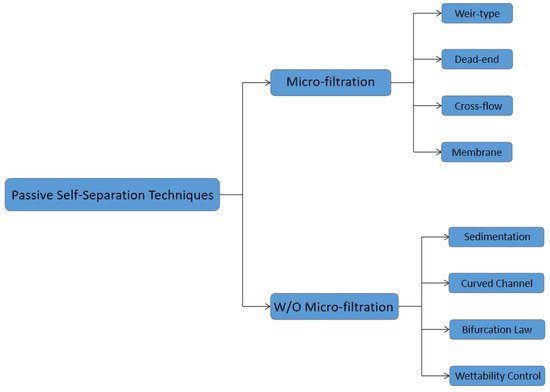
2. Passive Self-Separation with Filtration Using Micro-Structures
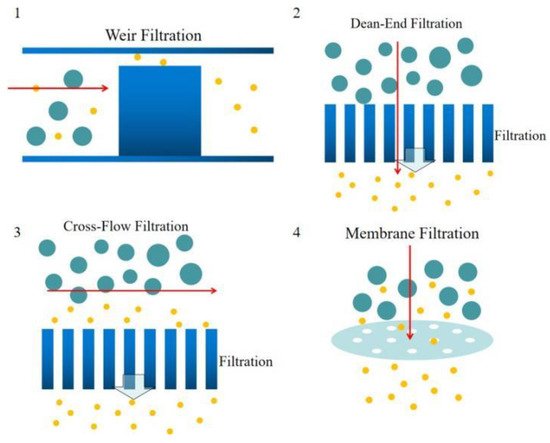
Weir-type microfiltration requires more steps in fabrication. To make a weir structure, additional soft lithography and etching processes are needed. Commonly, weir-type microfiltration can handle a limited amount of whole blood due to its tiny pore area compared to other types of microfiltration. Dead-end microfiltration mainly uses pore structures that are smaller than the blood cells dimensions to block blood cells and let the plasma continuously flow downstream. Once the blood cells accumulated and block all the pores, the microchannel will be clogged and stop separating plasma from whole blood. Cross-flow microfiltration can handle much more amount of whole blood. Due to the pillar filter structures or the pores on a membrane of the cross-flow microfiltration are located perpendicular to the main blood flow, blood cells could be easily removed by the main flow and not block the filtration pores. Lastly, membrane filtration is usually overpriced. The separation efficiency is mediocre, but the cost is high. In addition, it’s hard to control the pore size precisely and lead to a disappointing separation result.
3. Passive Self-Separation without Filtration



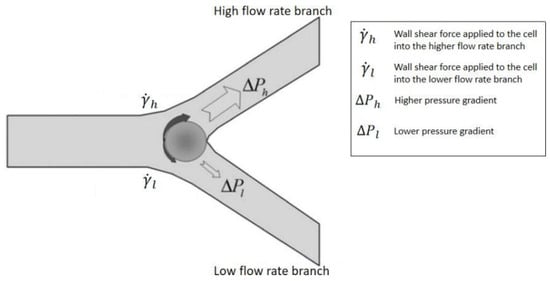
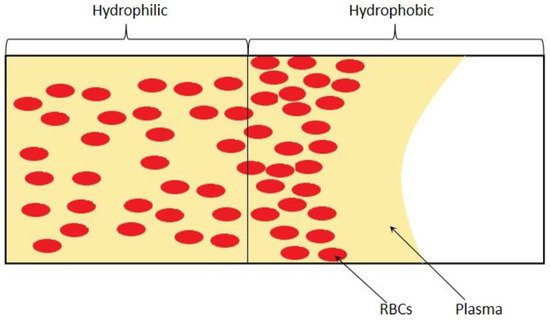
4. Future Trends
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering8070094
References
- Nunna, B.B.; Lee, E.S. Point-of-Care (POC) Micro Biochip for Cancer Diagnostics. In TechConnect Briefs 2017: Biomaterials and Biomedical, Proceedings of the TechConnect World Innovation Conference and Expo, Washington, DC, USA, 14–17 May 2017; Diagnostics and Bioimaging; Taylor Francis: Washington, DC, USA, 2017; Volume 3, Chapter 4; pp. 110–113. ISBN 978-0-9988782-0-1.
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Zhuang, S.; Lee, E.S. Sensitivity Study of Cancer Antigens (CA-125) Detection Using Interdigitated Electrodes Under Microfluidic Flow Condition. BioNanoScience 2019, 9, 203–214.
- Nunna, B.B.; Mandal, D.; Zhuang, S.; Lee, E.S. A standalone micro biochip to monitor the cancer progression by measuring cancer antigens as a point-of-care (POC) device for enhanced cancer management. In Proceedings of the 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT), Bethesda, MD, USA, 6–8 November 2017; pp. 212–215.
- Singh, H.; Zhuang, S.; Ingis, B.; Nunna, B.B.; Lee, E.S. Carbon-Based Catalysts for Oxygen Reduction Reaction: A Review on Degradation Mechanisms. Carbon 2019, 151, 160–174.
- Singh, H.; Zhuang, S.; Nunna, B.B.; Lee, E.S. Thermal Stability and Potential Cycling Durability of Nitrogen-Doped Graphene Modified by Metal-Organic Framework for Oxygen Reduction Reactions. Catalysts 2018, 8, 607.
- Zhuang, S.; Singh, H.; Nunna, B.B.; Mandal, D.; Boscoboinik, J.A.; Lee, E.S. Nitrogen-doped graphene-based catalyst with metal-reduced organic framework: Chemical analysis and structure control. Carbon 2018, 139, 933–944.
- Zhuang, S.; Nunna, B.B.; Lee, E.S. Metal-organic framework-modified nitrogen-doped graphene oxygen reduction re-action catalyst synthesized by nanoscale high-energy wet ball-milling structural and electrochemical characterization. MRS Commun. 2017, 8, 40–48.
- Kersaudy-Kerhoas, M.; Sollier, E. Micro-scale blood plasma separation: From acoustophoresis to egg-beaters. Lab Chip 2013, 13, 3323.
- Mahmoudi, G.; Babashkina, M.G.; Maniukiewicz, W.; Afkhami, F.A.; Nunna, B.B.; Zubkov, F.I.; Ptaszek, A.L.; Szczepanik, D.W.; Mitoraj, M.P.; Safin, D.A. Solvent-Induced Formation of Novel Ni(II) Complexes Derived from Bis-Thiosemicarbazone Ligand: An Insight from Experimental and Theoretical Investigations. Int. J. Mol. Sci. 2021, 22, 5337.
- Zhuang, S.; Nunna, B.B.; Mandal, D.; Lee, E.S. A Review of Nitrogen-Doped Graphene Catalysts for Proton Exchange Membrane Fuel Cells-Synthesis, Characterization, and Improvement. Nano-Struct. Nano-Objects 2017, 15, 140–152.
- Singh, H.; Zhuang, S.; Nunna, B.B.; Lee, E.S. Morphology and Chemical Structure of Modified Nitrogen-Doped Graphene for Highly Active Oxygen Reduction Reactions. In Proceedings of the 48th Power Source Conference, Denver, CO, USA, 11–14 June 2018.
- Nunna, B.B.; Zhuang, S.; Lee, E.S. Influence on Capillary Flow of Human Blood in PDMS Micro Channels due to various Surface Treatments, (ICNMM2016-8122). In Proceedings of the ASME 14th Int’l Conference on Nano-channels, Microchannels and Minichannels (ICNMM), Washington, DC, USA, 10–14 July 2016.
- Zhuang, S.; Nunna, B.B.; Boscoboinik, J.A.; Lee, E.S. Nitrogen-doped graphene catalysts: High energy wet ball milling synthesis and characterizations of functional groups and particle size variation with time and speed. Int. J. Energy Res. 2017, 41, 1–19.
- Mandal, D.; Nunna, B.B.; Zhuang, S.; Rakshit, S.; Lee, E.S. Carbon Nanotubes Based Biosensor for Detection of Cancer Antigens (CA-125) Under Shear Flow Condition. Nano-Struct. Nano-Objects 2017, 15, 180–185.
- Nunna, B.B.; Mandal, D.; Zhuang, S.; Lee, E.S. Innovative Point-of-Care (POC) Micro Biochip for Early Stage Ovarian Cancer Diagnostics. Sens. Transducers J. 2017, 214, 12–20.
- Zhuang, S.; Lei, L.; Nunna, B.B.; Lee, E.S. New Nitrogen-Doped Graphene/MOF-modified catalyst for Fuel Cell Systems. ECS Trans. 2016, 72, 149–154.
- Zhuang, S.; Lee, E.S.; Lei, L.; Nunna, B.B.; Kuang, L.; Zhang, W. Synthesis of Nitrogen-Doped Graphene Catalyst by High-Energy Wet Ball Milling for Electrochemical Systems. Int. J. Energy Res. 2016, 40, 2136–2149.
- Huang, C.-J.; Chen, Y.-H.; Wang, C.-H.; Chou, T.-C.; Lee, G.-B. Integrated microfluidic systems for automatic glucose sensing and insulin injection. Sens. Actuators B Chem. 2007, 122, 461–468.
- Pei, J.; Tian, F.; Thundat, T. Glucose Biosensor Based on the Microcantilever. Anal. Chem. 2004, 76, 292–297.
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120.
- Oh, Y.K.; Joung, H.-A.; Kim, S.; Kim, M.-G. Vertical flow immunoassay (VFA) biosensor for a rapid one-step immunoassay. Lab Chip 2013, 13, 768.
- Liu, S.; Su, W.; Ding, X. A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection. Sensors 2016, 16, 2086.
- Arif, T.M.; Ji, Z.; Rahim, M.A.; Nunna, B.B. Modeling Focused-Ultrasound Response for Non-Invasive Treatment Using Machine Learning. Bioengineering 2021, 8, 74.
- Turgeon, M.L. Clinical Hematology: Theory and Procedures; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005.
- Paulus, J. Platelet size in man. Blood 1975, 46, 321–336.
- Lewis, S.M.; Bain, B.J.; Bates, I.; Dacie, J.V. Dacie and Lewis Practical Haematology; Churchill Livingstone/Elsevier: London, UK, 2006.
- Fung, Y.C. 1981 Biomechanics—Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 1993.
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Singh, H.; Zhuang, S.; Misra, D.; Bhuyian, N.U.; Lee, E.S. Detection of Cancer Antigens (CA-125) using Gold Nano Particles on Interdigitated Electrode based Microfluidic Biosensor. Nano Converg. 2019, 6.
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Zhuang, S.; Lee, E.S. Hemorheology in PDMS Microchannel with Varied Surface Roughness APS Meeting Abstracts 2015. Available online: https://ui.adsabs.harvard.edu/abs/2015APS..DFDKP1116N/abstract (accessed on 3 July 2021).
- Nunna, B.B.; Zhuang, S.; Javier, J.; Mandal, D.; Lee, E.S. Biomolecular Detection using Molecularly Imprinted Polymers (MIPs) at Point-of-Care (POC) Micro Biochip. In Proceedings of the 2016 IEEE-NIH 2016 Healthcare Innovation Point of Care Technologies Conference HI POCT16, (PCHT16-0099), Cancun, Mexico, 9–11 November 2016.
- Zhuang, S.; Nunna, B.B.; Lei, L.; Lee, E.S. Synthesis of Nitrogen-doped Graphene Catalyst by Wet Ball Milling for Electrochemical Systems, (Paper ID: 2425505). In Proceedings of the 251st ACS National Meeting Exposition, San Diego, CA, USA, 13–17 March 2016.
- Nunna, B.B.; Zhuang, S.; Malave, I.; Lee, E.S. Ovarian Cancer Diagnosis using Micro Biochip. In Proceedings of the NIH-IEEE 2015 Strategic Conference on Healthcare Innovations and Point-of-Care Technologies for Precision Medicine, (PCHT15-0056), Bethesda, MD, USA, 9–10 November 2015.
- Lee, E.S.; Nunna, B.B.; Suh, S.K. Microfluidic Diagnostic Assembly. U.S. Patent and Trademark Office. U.S. Patent US10898894B2, 26 January 2021. Available online: https://patents.google.com/patent/US10898894B2/en. (accessed on 3 July 2021).
- Lee, E.S.; Nunna, B.B. Biomarker Detection and Self-Separation of Serum During Capillary Flow. U.S. Patent and Trademark Office. U.S. Patent US10481154B2, 19 November 2019. Available online: https://patents.google.com/patent/US10481154B2/en. (accessed on 3 July 2021).
- Lee, E.S.; Nunna, B.B. Enhanced Sensitivity and Specificity for Point-Of-Care (POC) Micro Biochip. U.S. Patent and Trademark Office. U.S. Patent US20200182864A1, 11 June 2020. Available online: https://patents.google.com/patent/US20200182864A1/en. (accessed on 3 July 2021).
- Lee, E.S.; Nunna, B.B. Microfluidic Biochip with Enhanced Sensitivity. U.S. Patent and Trademark Office. U.S. Patent US11020740B2, 1 June 2021. Available online: https://patents.google.com/patent/US11020740B2/en. (accessed on 3 July 2021).
- Yang, C.-H.; Hsieh, Y.-L.; Tsou, P.-H.; Li, B.-R. Thermopneumatic suction integrated microfluidic blood analysis system. PLoS ONE 2019, 14, e0208676.
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506.
- Lenshof, A.; Laurell, T. Continuous separation of cells and particles in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1203.
- Das, C.M.; Becker, F.; Vernon, S.; Noshari, J.; Joyce, C.; Gascoyne, P.R. Dielectrophoretic Segregation of Different Human Cell Types on Microscope Slides. Anal. Chem. 2005, 77, 2708–2719.
- Szydzik, C.; Khoshmanesh, K.; Mitchell, A.; Karnutsch, C. Microfluidic platform for separation and extraction of plasma from whole blood using dielectrophoresis. Biomicrofluidics 2015, 9, 064120.
- Nakashima, Y.; Hata, S.; Yasuda, T. Blood plasma separation and extraction from a minute amount of blood using dielectrophoretic and capillary forces. Sens. Actuators 2010, 145, 561–569.
- Macdonald, M.P.; Spalding, G.C.; Dholakia, K. Microfluidic sorting in an optical lattice. Nature 2003, 426, 421–424.
- Huh, D.; Bahng, J.H.; Ling, Y.; Wei, H.-H.; Kripfgans, O.D.; Fowlkes, J.B.; Grotberg, J.B.; Takayama, S. Gravity-Driven Microfluidic Particle Sorting Device with Hydrodynamic Separation Amplification. Anal. Chem. 2007, 79, 1369–1376.
- Lee, B.S.; Lee, J.-N.; Park, J.-M.; Lee, J.-G.; Kim, S.; Cho, Y.-K.; Ko, C. A fully automated immunoassay from whole blood on a disc. Lab Chip 2009, 9, 1548.
- Jung, J.; Han, K.-H. Lateral-driven continuous magnetophoretic separation of blood cells. Appl. Phys. Lett. 2008, 93, 223902.
- Liao, S.-H.; Chang, C.-Y.; Chang, H.-C. A capillary dielectrophoretic chip for real-time blood cell separation from a drop of whole blood. Biomicrofluidics 2013, 7, 024110.
- Yan, S.; Zhang, J.; Alici, G.; Du, H.; Zhu, Y.; Li, W. Isolating plasma from blood using a dielectrophoresis-active hydrophoretic device. Lab Chip 2014, 14, 2993.
- Yeo, L.Y.; Friend, J.R.; Arifin, D.R. Electric tempest in a teacup: The tea leaf analogy to microfluidic blood plasma separation. Appl. Phys. Lett. 2006, 89, 103516.
- Li, Y.; Dalton, C.; Crabtree, H.J.; Nilsson, G.; Kaler, K.V. Continuous dielectrophoretic cell separation microfluidic device. Lab Chip 2007, 7, 239–248.
- Grady, M.; Pineau, M.; Pynes, M.K.; Katz, L.B.; Ginsberg, B. A Clinical Evaluation of Routine Blood Sampling Practices in Patients with Diabetes. J. Diabetes Sci. Technol. 2014, 8, 691–698.
- Colace, T.V.; Tormoen, G.W.; Mccarty, O.J.T.; Diamond, S.L. Microfluidics and Coagulation Biology. Annu. Rev. Biomed. Eng. 2013, 15, 283–303.
- Ingis, B.; Lee, E.S. 3D Printing for Whole Blood Filters Designed for Simple Integration with a Variety of Sensor Platforms. In Proceedings of the IEEE-NIH 2019 Healthcare Innovations and Point-of-Care Technologies (HI-POCT2019), Bethesda, MD, USA, 20–22 November 2019.
- Warkiani, M.E.; Tay, A.K.P.; Guan, G.; Han, J. Membrane-less microfiltration using inertial microfluidics. Sci. Rep. 2015, 5, 11018.
- Pamme, N. Continuous flow separations in microfluidic devices. Lab Chip 2007, 7, 1644.
- Meena, G.G.; Jain, A.; Parks, J.W.; Stambaugh, A.; Patterson, J.L.; Hawkins, A.R.; Schmidt, H. Integration of sample preparation and analysis into an optofluidic chip for multi-target disease detection. Lab Chip 2018, 18, 3678–3686.
- Lee, K.K.; Ahn, C.H.T. A new on-chip whole blood/plasma separator driven by asymmetric capillary forces. Lab Chip 2013, 13, 3261.
- Bellanger, H.; Darmanin, T.; Taffin De Givenchy, E.; Guittard, F. Chemical and Physical Pathways for the Preparation of Superoleophobic Surfaces and Related Wetting Theories. Chem. Rev. 2014, 114, 2694–2716.
- Ji, H.M.; Samper, V.; Chen, Y.; Heng, C.K.; Lim, T.M.; Yobas, L. Silicon-based microfilters for whole blood cell separation. Biomed. Microdevices 2008, 10, 251–257.
- Nam, Y.; Kim, M.; Kim, T. Pneumatically controlled multi-level microchannel for separation and extraction of microparticles. Sens. Actuators Chem. 2014, 190, 86–92.
- Wilding, P.; Kricka, L.J.; Cheng, J.; Hvichia, G.; Shoffner, M.A.; Fortina, P. Integrated cell isolation and polymerase chain reaction analysis using silicon microfilter chambers. Anal. Biochem. 1998, 257, 95–100.
- Zheng, S.; Lin, H.; Liu, J.-Q.; Balic, M.; Datar, R.; Cote, R.J.; Tai, Y.-C. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 2007, 1162, 154–161.
- Vandelinder, V.; Groisman, A. Separation of Plasma from Whole Human Blood in a Continuous Cross-Flow in a Molded Microfluidic Device. Anal. Chem. 2006, 78, 3765–3771.
- Didar, T.F.; Li, K.; Tabrizian, M.; Veres, T. High throughput multilayer microfluidic particle separation platform using embedded thermoplasticbased micropumping. Lab Chip 2013, 13, 2615.
- Didar, T.F.; Li, K.; Veres, T.; Tabrizian, M. Separation of rare oligodendrocyte progenitor cells from brain using a high-throughput multilayer thermoplastic-based microfluidic device. Biomaterials 2013, 34, 5588–5593.
- Hosokawa, M.; Yoshikawa, T.; Negishi, R.; Yoshino, T.; Koh, Y.; Kenmotsu, H.; Naito, T.; Takahashi, T.; Yamamoto, N.; Kikuhara, Y.; et al. Microcavity Array System for Size-Based Enrichment of Circulating Tumor Cells from the Blood of Patients with Small-Cell Lung Cancer. Anal. Chem. 2013, 85, 5692–5698.
- Dean, W.R., XVI. Note on the motion of fluid in a curved pipe. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1927, 4, 208–223.
- Dean, W.R. The stream-line motion of fluid in a curved pipe (Second paper). Lond. Edinb. Dublin Philos. Mag. J. Sci. 1928, 5, 673–695.
- Nivedita, N.; Papautsky, I. Continuous separation of blood cells in spiral microfluidic devices. Biomicrofluidics 2013, 7, 054101.
- Brigden, M.L. Clinical utility of the erythrocyte sedimentation rate. Am. Fam. Physician. 1999, 60, 1443–1450.
- Huang, C.-T.; Li, P.-N.; Pai, C.-Y.; Leu, T.-S.; Jen, C.-P. Design and Simulation of a Microfluidic Blood-Plasma Separation Chip Using Microchannel Structures. Sep. Sci. Technol. 2009, 45, 42–49.
- Dimov, I.K.; Basabe-Desmonts, L.; Garcia-Cordero, J.L.; Ross, B.M.; Ricco, A.J.; Lee, L.P. Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS). Lab Chip 2011, 11, 845–850.
- Fung, Y.C.; Zweifach, B.W. Microcirculation: Mechanics of Blood Flow in Capillaries. Annu. Rev. Fluid Mech. 1971, 3, 189–210.
- Yang, S.; Undar, A.; Zahn, J.D. A microfluidic device for continuous, real time blood plasma separation. Lab Chip 2006, 6, 871–880.
- Xue, X.; Patel, M.K.; Kersaudy-Kerhoas, M.; Bailey, C.; Desmulliez, M.P. Parametrical modeling and design optimization of blood plasma separation device with microchannel mechanism. In Proceedings of the 59th Electronic Components and Technology Conference 2009, San Diego, CA, USA, 26–29 May 2009; pp. 1970–1976.
- Nunna, B.B.; Zhuang, S.; Lee, E.S. Flow control mechanism of capillary driven flow in microchannel using non-mechanical forces. In Proceedings of the Aps Division of Fluid Dynamics abstract id. A25.002, Portland, OR, USA, 20–26 November 2016.
- Pitt, W.G.; Alizadeh, M.; Blanco, R.; Hunter, A.K.; Bledsoe, C.G.; McClellan, D.S.; Beard, W.C.; Jacob, R.S.; Carter, A.; Anderson, C.M.; et al. Factors affecting sedimentational separation of bacteria from blood. Biotechnol. Prog. 2020, 36, e2892.
- Maria, M.S.; Rakesh, P.E.; Chandra, T.S.; Sen, A.K. Capillary flow of blood in a microchannel with differential wetting for blood plasma separation and on-chip glucose detection. Biomicrofluidics 2016, 10, 054108.
- Shaw, J.L.V. Practical challenges related to point of care testing. Pract. Lab. Med. 2016, 4, 22–29.
