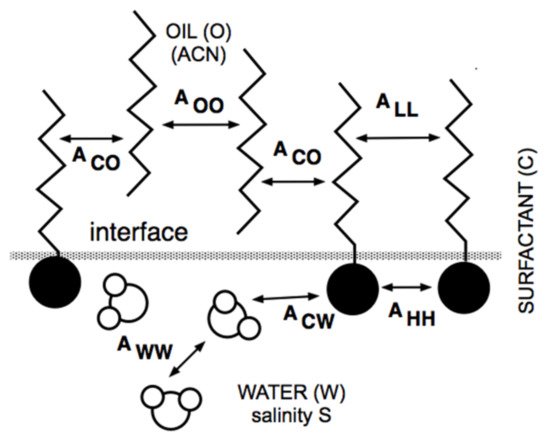
Extended surfactants are molecules including an intramolecular extension that allow attaining high performance without the need for cosurfactant or linker alcohol. The polypropylene oxide chain intramolecular extension generates a polarity transition inside the molecule that produces more interactions with the oil and aqueous phases. The idea was developed in the 1990s, basically to fasten together the rather hydrophilic surfactant and the lipophilic linker, producing the same effect as the mixture without losing a part of the lipophilic linker going away from the interface. Since the lipophilic linker was an amphiphile with a small hydrophilic part located close to the interface, the single structure was developed to imitate the mixture situation. It contains a polar head located in water, then an intermediate slightly polar zone in the oil phase close to the interface, and finally, the surfactant classical hydrocarbon tail.
- formulation
- normalized hydrophilic–lipophilic deviation HLDN
- extended surfactant
- solubilization
- enhanced oil recovery
- interfacial tension
1. Introduction
Table 1. Molecular structure and classification of sulfate head extended surfactants according to its normalized characteristic parameter (SCPN) [2]
-
Extended Surfactant 1
σ
k
SCPN= σ/k*
Author and year
Ref.
S/12/6/2/SO4
-1.43
0.075
-19.1
Miñana-Perez, 1995
[13]
S/12/10/2/SO4
-0.3
0.11
-2.7
Miñana-Perez, 1995
[13]
S/12/14/2/SO4
1.21
0.16
7.6
Miñana-Perez, 1995
[13]
A/14-15/8/0/SO4
0.16
0.13
1.2
Witthayapanyanon, 2006
[30]
A/10/18/2/SO4
0.57
0.053
10.8
Do, 2009
[39]
A/14-15/4/0/SO4
-0.18
0.11
-1.6
Velásquez, 2010
[24]
A/16-17/4/0/SO4
-0.29
0.11
-2.6
Velásquez, 2010
[24]
A/12-13/8/0/SO4
-0.52
0.08
-6.5
Velásquez, 2010
[24]
A/12-13/4/0/SO4
-0.98
0.11
-8.9
Velásquez, 2010
[24]
Chen/8/9/3/SO4
-0.39
0.17
-2.3
Chen, 2019
[40]
A/12-13/4/0/SO4
-1.55
0.049
-31.6
Wang, 2019
[41]
He/13/2/0/SO4
-1.8
0.056
-32.1
He, 2019
[42]
A/10/4/0/SO4
-2.24
0.053
-42.3
Phaodee, 2020
[43]
1Nomenclature: A: Alfoterra, S: Seppic, Chen and He are first authors of the papers where these surfactants were synthetized. A/10/18/2/S stands for Alfoterra/C10/PO18/O2/SO4. This is the same nomenclature as [2]
*SCPN is the surfactant classification parameter. Higher SCPN indicates a more important lipophilic part of the molecule (hydrocarbon tail and PO extension), a lower SCPN (more negative) indicates a more important hydrophilic head contribution.
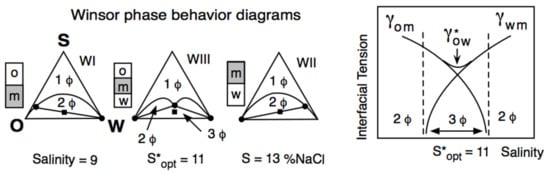
2. Historical Introduction on Formulation Concepts
The Unidimensional Scan of a Formulation Variable
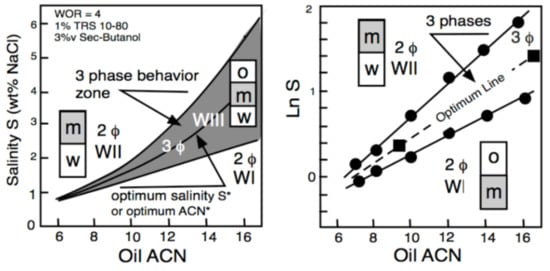
3. Multivariable Scans and Generalized HLD Expression for Optimum Formulation
| HLD Equation–Surfactant Type |
|---|
| ΔHLD1 = ΔLnS − 0.16 ΔACN = 0 for alkylbenzene sulfonates ΔHLD2 = ΔLnS − 0.19 ΔACN = 0 for alkyltrimethyl ammonium chlorides ΔHLD3 = ΔLnS − 0.07 ΔACN = 0 for alkyl hexapropyleneoxide diethylenoxide sulfates ΔHLD4 = 0.33 ΔSAT − ΔEON = 0 for ethoxylated n-alcohol with EON~5 and T~25 °C ΔHLD5 = 0.13 ΔS − ΔEON = 0 for ethoxylated n-alcohol with EON~5 and T~25 °C ΔHLD6 = 2.25 ΔSAT − ΔACN = 0 for ethoxylated n-alcohol with EON~5 ΔHLD7 = − 0.24 ΔACN − ΔEON = 0 for ethoxylated n-alcohol with EON~5 ΔHLD8 = − ΔT − 20 ΔACN = 0 for n-alkyl sulfates ΔHLD9 = − ΔT − 14.3 ΔACN = 0 for alkylbenzene sulfonates ΔHLD10 = ΔT − 4 ΔACN = 0 for ethoxylated nonionic (EON~5–6 & T~20–30 °C) ΔHLD11 = ΔT − 1.4 ΔACN = 0 for ethoxylated nonionic (EON~8–9 & T~70 °C) ΔHLD12 = ΔT − 0.90 ΔACN = 0 for ethoxylated nonionic (EON~10–11 & T~80–90 °C) ΔHLD13 = − ΔGN − 0.12 ΔACN = 0 for polyglyceryl monolaurate (GN~5–6) ΔHLD14 = ΔLnS − 0.14 ΔPON = 0 for alkyl polypropyleneoxide diethylenoxide sulfates |
where S is the salinity in wt% NaCl, EON is the exact or average number of ethylene oxide groups, SAT is the surfactant n-alkyl tail length in carbon atom number, T is the temperature in °C, and GN is the number of glyceryl group in polyglyceryl monolaurate oligomers.
3.1. The Normalized Hydrophilic Lipophilic Deviation (HLDN) Equation
| HLDN Equation−Surfactant Type |
|---|
| ΔHLDN1 = 6.25 ΔLnS − ΔACN = 0 for alkylbenzene sulfonates ΔHLDN2 = 5.26 ΔLnS − ΔACN = 0 for alkyltrimethyl ammonium chlorides ΔHLDN3 = 14.3 ΔLnS − ΔACN = 0 for alkyl hexapropyleneoxide diethylenoxide sulf. ΔHLDN4 = 1.4 ΔSAT − 4.2 ΔEON = 0 for ethoxylated n-alcohol (EON~5 & T~25 °C) ΔHLDN5 = 0.55 ΔS − 4.2 ΔEON = 0 for ethoxylated n-alcohol (EON~5 & T~25 °C) ΔHLDN6 = 2.25 ΔSAT − ΔACN = 0 for ethoxylated n-alcohol with EON~5 ΔHLDN7 = − 4.2 ΔEON − ΔACN = 0 for ethoxylated n-alcohol with EON~5 ΔHLDN8 = − 0.05 ΔT − ΔACN = 0 for n-alkyl sulfates ΔHLDN9 = − 0.07 ΔT − ΔACN = 0 for alkylbenzene sulfonates ΔHLDN10 = 0.25 ΔT − ΔACN = 0 for ethoxylated nonionic (EON~5–6 & T~20–30 °C) ΔHLDN11 = 0.70 ΔT − ΔACN = 0 for ethoxylated nonionic (EON~8–9 & T~70 °C) ΔHLDN12 = 1.1 ΔT − ΔACN = 0 for ethoxylated nonionic (EON~11 & T~80–90 °C) ΔHLDN13 = − 8.3 ΔGN − ΔACN = 0 for polyglyceryl monolaurate (GN~5–6) ΔHLDN14 = 1.2 ΔPON − ΔACN = 0 for alkyl polypropyleneoxide PON sulfates |
3.2. The Normalized Surfactant Characteristic Parameter (SCPN)
4. Lipophilic and Hydrophilic Linkers, and Extended Surfactants
4.1. The Lipophilic Linker
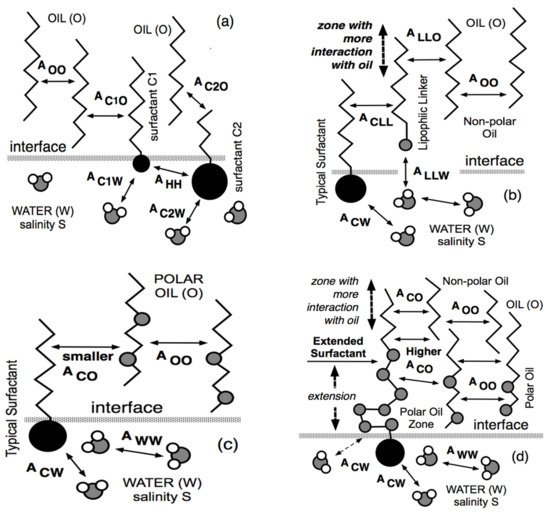
4.2. The Hydrophilic Linker
4.3. The Extended Surfactant with an Intramolecular PO Extension
4.4. The Increased Performance of Extended Surfactant Systems with Polar Oils and Crude Oils
This entry is adapted from the peer-reviewed paper 10.3390/molecules26123771
