Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, General & Internal
Melatonin is a pleotropic molecule with numerous biological activities. Epidemiological and experimental studies have documented that melatonin could inhibit different types of cancer in vitro and in vivo.
- pineal gland
- anticancer
- cancer therapy
- hormonal therapy
- phytomelatonin
1. Melatonin Biosynthesis and Metabolism in Human Body
Melatonin was isolated in 1958, by the dermatologist Aaron Lerner, from bovine pineal gland. Although it is mainly secreted from the pineal gland, there are many other secondary sources including; retina, gut, skin, platelets and bone marrow, and probably other structures, but their systemic contribution is insignificant [1].
The starting material of melatonin biosynthesis in humans is tryptophan, an essential amino acid. Through the action of tryptophan hydroxylase (TP5H) and aromatic acid decarboxylase (AADC), enzymes tryptophan is converted to the neurotransmitter, serotonin. In the subsequent step, serotonin is converted into melatonin through the influence of arylalkylamine N-acetyltransferase (AANAT) and hydroxyindole-O-methyltransferase (HIOMT) enzymes [2] (Figure 1).
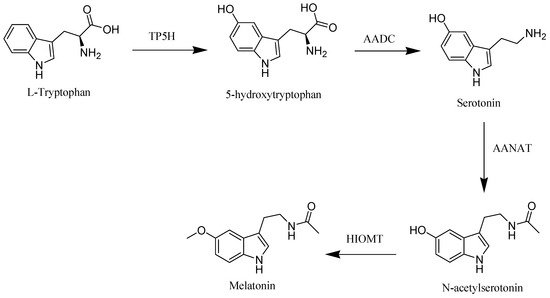
Figure 1. Melatonin biosynthesis in human.
Melatonin is not stored inside the pineal gland and it is released as it is synthetized, so the plasma hormone profile faithfully reflects the pineal activity [3]. Moreover, amphiphilic nature and the small size of melatonin facilitates its passage across cell membranes and its access to various fluids, tissues, and cellular compartments as saliva, urine, cerebrospinal fluid, preovulatory follicle, semen, amniotic fluid, and milk [1][4][5].
Melatonin is metabolized mainly by cytochrome P450 in the liver. It has been demonstrated that melatonin was metabolized to 6-hydroxymelatonin and N-acetylserotonin by CYP1A1 and CYP 2C19, respectively, at Phase I metabolism, and most of them were subsequently converted to sulfate conjugates by sulfotransferases in human liver and excreted in the urine [6]. A small portion of melatonin is degraded by other tissues including skin and brain by either CYPA2B or 2,3-indolamine dioxygenase to form 6-hydroxymelatonin or N1-acetyl-N2-formyl-5-methoxykynurenine (AFMK). The urinary excretion probably is not the major metabolic route of AFMK judging from its water solubility [7].
Melatonin has specific receptors to regulate many physiological functions namely; MT1 and MT2, both are members of the seven transmembrane G-protein coupled receptor family [8]. Human MT1 and MT2 receptors are 350 and 362 amino acids long, respectively, with molecular weights of 39–40 kDa and 55% amino acid homology overall [9]. Both MT1 and MT2 affect protein kinase activity through inhibition of adenylyl (cAMP) and guanylyl (cGMP) cyclase, respectively. Furthermore, activation of the phospholipase C pathway that leads to increase in inositol triphosphate (IP3) and 1, 2-diacylglycerol (DAG) levels has been proved for both MT1 and MT2 receptors [10].
2. Biological Activities of Melatonin
Melatonin is widespread in nature, and it plays a vital role in different biological activities [11]. A study has been carried out in aged animals that showed melatonin’s effect on body temperature and energy balance [12]. Several studies have shed light on the melatonin immunomodulatory effect. It was reported that melatonin may regulate the activation of T/B cells in pinealectomy mice in a dose-dependent manner [13]. Besides, it shows immunomodulation and neuroprotective potential in a pharmacological Alzheimer’s disease mouse model [14]. Moreover, melatonin was known to be associated with bone homeostasis. Administration of melatonin exhibited a promising strategy to manage postmenopausal patients via restoring the osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells [15]. It also maintains bone balance; increases the osteogenic differentiation of bone marrow mesenchymal stem cells, and suppresses osteoclastogenesis [16]. A recent clinical trial has investigated the effect of melatonin consumption on controlling arterial pressure and anthropometric indices in type 2 diabetes mellitus patients. It reduced significantly the mean level of systolic pressure, mean arterial pressure, pulse pressure, and conicity index in the intervention group [17]. In addition, the chronobiotic properties of melatonin have been evaluated. It revealed that the administration of melatonin may regulate sleep disorders related to abnormal timing of the circadian system: jetlag, shift work, delayed sleep phase syndrome, and some elderly sleep difficulties [18]. Additionally, melatonin was able to inhibit neuroinflammation and relieve depression by autophagy modulation through FOXO3a signaling [19]. Recently, melatonin has been investigated as a candidate drug for the management of corona virus infection. It docks with novel coronavirus proteins and exhibits a variety of interactions with an interesting docking score that leads to prevent the virus proteins, which lead to demolish the virus as well [20].
3. The Use of Melatonin in Cancer Treatment
Plethora of clinical research have reported the oncostatic role of melatonin against various types of cancer such as Gastric cancer [21][22][23], breast cancer [24][25][26], oral cancer [27][28], prostate cancer [29][30][31], and other more types.
3.1. Gastric Cancer
Gastric cancer (stomach cancer) is one of the most common cancers worldwide. According to GLOBOCAN 2018 data, gastric cancer is the 3rd most deadly cancer [32]. Melatonin has been reported with distinguished anticancer activity against gastric cancer. The anti-gastric cancer mechanisms of melatonin are still not fully understood, however, various studies suggested several mechanisms for anticancer activity of melatonin including stimulation of immunity, cell proliferation inhibiting, and apoptosis induction [33][34]. Zhang et al. have investigated the impact of melatonin on the functions of gastric adenocarcinoma cell line, SGC7901, including apoptosis, cell proliferation, cell migration, and colony formation. They demonstrated that melatonin could inhibit colony formation, cell proliferation, cell migration, and enhanced apoptosis [21]. In another study on SCG7901 human gastric cells, Wang et al. have illustrated the association of melatonin with RZR/RORγ pathway under hypoxia. Their results showed suppression in the activity of RZR/RORγ, in addition to suppression in SUMO-specific protease 1 (SENP1) signaling pathway, which is crucial for stabilizing the hypoxia inducible factor-1α (HIF 1α) during hypoxia in response to melatonin. Moreover, melatonin was able to reduce the vascular endothelial growth factor (VEGF) expression and suppress metastasis [22]. In agreement with this, Wang et al. followed up with another study to evaluate the anticancer activity of melatonin on the growth and angiogenesis of SGC7901cells, revealing the inhibitory effect of melatonin on the growth of SGC7901cells. The low concentration of melatonin (0.01, 0.1, and 1 mM) had no clear impact on VEGF secretions, however, higher concentration (3 mM) had clearly suppressed VEGF secretions. Besides, the expression of melatonin nuclear receptor RZR/RORγ, HIF-1α, SUMO-specific protease 1, and VEGF had been reduced within SGC7901 during tumorigenesis in response to treatment with melatonin [35]. In addition, Song et al. have investigated the effect of melatonin on SGC7901 cells in term of protein production using protein chip technology. Melatonin was found to induce cell cycle arrest. Furthermore, melatonin induced changes in proteins that are related to cell proliferation and apoptosis represented in downregulation of phospho-CDC25A, CDC25A, p21, phosphor-p21, and Bcl-xl, upregulation of Bax, an activation of caspase-3 and an increase in the level of cleaved caspase-9, which ensured the implication of mitochondria in melatonin-induced [36].
3.2. Glioblastoma
Glioblastoma is the most common and aggressive primary brain tumor in adults. The incidence rate of glioblastoma is 5–8 per 100,000, representing around 54% of diagnosed gliomas cases. Glioblastoma has short life expectancy, less than one year since diagnosis in average, which is owed to the tumor recurrences high rate [37][38]. Glioblastoma was reported with higher frequency and 1.6 higher incidences in males as compared to females [38][39]. Glioma stem-like cells are subpopulation in glioblastoma, they play a crucial role in the tumor growth maintenance and recurrence [40][41][42], and promote self-renewing capacity and tumor propagation [43][44][45]. Melatonin showed an anticancer effect against glioblastoma, and it was also reported to overcome the multi-drug resistance in glioblastoma treatment [46][47][48]. Sung et al. recently have investigated the impact of combination of melatonin with vorinostat on the expression of transcription factor EB and apoptosis in glioblastoma cells and glioma cancer stem cells. The expression of transcription factor EB, which needs oligomerization to regulate transcription, was reported to be increased in glioblastoma. The combination of vorinostat and melatonin induce a downregulation of the transcription factor EB and oligomerization, which increased apoptosis related gens, hence, cells apoptosis was activated [49]. In another study, Chen et al. have studied the roles of melatonin and the associated mechanisms against glioblastoma stem-like cells. Their results demonstrated that melatonin altered the glioblastoma stem-like cells biology and inhibited glioblastoma stem-like cells proliferation. Moreover, melatonin showed to alter the transcription factors profile inhibiting the initiation and propagation of tumor. In addition to the impairment of EZH2–STAT3 interaction and EZH2 S21 phosphorylation, melatonin has multiple roles in attenuating several key signals related to survival and self-renewal in glioblastoma stem-like cells [46]. Lai et al. have studied the microenvironment of glioma investigating the correlation of melatonin treatment and molecular markers in glioblastoma multiform including SIRT1, CCL2, ICAM-1, and VCAM-1. Their results showed melatonin administration increased the expression of SIRT1, which inhibit the growth and proliferation of glioma cells [50]. In another recent study, Fernandez-Gil et al. have explored whether treatment with melatonin can restore the oxidative phosphorylation after metabolic switch to glycolysis in glioblastoma cells. The results showed that melatonin significantly decreased the viability and inhibited the proliferation of glioblastoma cells. Besides, it modulates a metabolic shift from glycolysis to oxidative phosphorylation, which lead to a reduction in the malignant properties of glioblastoma cells [51]. Additionally, it was reported that the melatonin antitumor effect can be through suppression of the EZH2-NOTCH1 signaling axis in glioblastoma stem-like cells [52]. Moreover, several studies have shown the melatonin impact on glioblastoma cells via enhancing apoptosis and inhibiting cell migration and invasion [53][54][55].
3.3. Prostate Cancer
Prostate cancer (PC) is the most common cancer in males. It is the fifth leading cause of death in men cancer cases worldwide [56][57]. Prostates represent a target for melatonin which has been proven with its inhibitory effect on the cell growth of prostate cancer [58][59][60]. Wang et al. have investigated the effect of melatonin on prostate cancer cells. Their results showed that melatonin downregulated the expression of matrix metallopeptidase 13 (MMP-13) and inhibited the invasive and migratory capacities in prostate cancer cells via the phospholipase C, p38, and c-Jun signaling cascades and MT1 receptor. MMP-13 have been reported to be highly expressed in prostate cancer patients as compared to healthy individuals. Moreover, melatonine suppressed the growth rate and metastasis in prostate cancer cells in both in vivo and in vitro models [61]. In a retrospective study, Zharinov et al. have evaluated the use of melatonin in prostate cancer patients with different risk groups showing that there is no significant difference between the melatonin-treated and not treated in the favorable and intermediate prognoses groups. However, an increase in the survival rate in poor prognosis group has been demonstrated in melatonin-treated patients as compared to untreated patients [62]. Liu et al. investigated the melatonin activity in 22Rv1 and LNCaP prostate cancer cells. They showed that these cells overexpress androgen receptor splice variant-7 (AR-V7) and activate nuclear factor-kappa B (NF-κB) that results in upregulation of the expression of IL-6. Melatonin showed inhibitory effect on expression of AR-V7 and its induced activation of NF-κB and IL-6 gene transcription [63]. Besides, Guilherme et al. have evaluated the activity of melatonin alone or combined with docosahexaenoic acid on PNT1A prostate cancer cells in regard to proliferation relevant pathways, ROS production, and mitochondria bioenergetics. Melatonin upon coincubation with docosahexaenoic acid improved the oxidative phosphorylation and restored mitochondrial bioenergetic reserve capacity. These melatonin induced alterations were related to AKT/mTOR dephosphorylation, and modulation of ERK1/2 expression [64]. An in vivo study has demonstrated the antitumor effect of melaonin on prostate cancer [31]. Moreover, melatonin inhibited angiogenesis in prostate cancer cells via amplifying the miRNA3195 and miRNA374b expression [65]. It also inhibited cell growth in LNCap and PC-3 cell line [29].
3.4. Lung Cancer
In cancer related-deaths globally, lung cancer is one of the most common type that is well known with its strong metastasis [66][67]. It is the second most common cancer in males and females according to the American Cancer Society (ACS) [68]. Melatonin has shown its effectiveness against lung cancer [69][70][71]. Recently, Ma et al. have studied the effect of melatonin on non-small cell lung cancer. Melatonin administration remarkably enhanced apoptosis, in addition to inhibition of proliferation, invasion, and metastasis in NSCLC. In addition, melatonin reduced the level of HDAC9 in NSCLC [69]. In another study, Yun et al. have investigated the effect of administration of melatonin in combination with gefitinib in H1975 NSCLC and HCC827 lung tumor cell line. The results showed that co-administration of melatonin with gefitinib reduced the viability of H1975 cells with harbored T790M somatic mutation, as compared to HCC827 cells with an active epidermal growth factor receptor (EGFR) mutation. This decreased viability and cell death lead to reduced phosphorylation of EGFR and Akt, in turn, decreasing the expression of several survival proteins; such as Bcl-xL, Bcl-2, and surviving, and activating caspase 3 in H1975 cells. Additionally, it was found that co-administration induced apoptosis and downregulated EGFR phosphorylation in H1975 as compared to administration of melatonin or gefitinib alone, suggesting that melatonin acts by increase the sensitivity of H1975 cells to gefitinib [70]. Furthermore, Plaimee et al. have evaluated the anticancer effect of melatonin in combination with cisplastin in SK-LU-1, human lung adenocarcinoma cisplatin-sensitive cell line. The results showed that co-administration of melatonin decreased the IC50 of cisplatin and enhanced apoptosis of SK-LU-1 cells via increasing the membrane polarization of mitochondria, activating caspases-3/7, and promoting cell cycle arrest, as compared to using cisplatin alone [71]. Besides, Zhou et al. have explored the anticancer effect of melatonin and its mechanism on A549 cells, human lung adenocarcinoma cell line. Treatment with melatonin decreased the viability and inhibited migration of A549 cells. Moreover, downregulation of the expression of MLCK and OPN have been observed, in addition to a reduction in phosphorylation of MLC of A549 cells. However, an elevation in the occludin expression involving JNK/MAPK pathway have been demonstrated suggesting that these effects mediate inhibition of the migration of A549 [72].
3.5. Ovarian Cancer
Ovarian cancer is the main cause of death worldwide among the gynecological malignancies [73]. Melatonin has been reported with its efficiency against ovarian cancer [74][75]. Chuffa et al. have studied the anti-inflammatory activity of melatonin in modulation of toll-like receptors (TLR) which expressed on the surface of ovarian cancer. The results showed that there is no decrease in the level of TLR2 in response to melatonin. However, the ovarian cancer-associated increase in several proteins was suppressed by melatonin. Moreover, melatonin decrease the expression of IRF-3, IkBα, TRIF, p65, and NF-kB, which are involved in TLR4 mediated signaling pathway, suggesting the role of melatonin in attenuating the TLR4-mediated TRIF- and MyD88-dependent signaling pathways in ovarian cancer in ethanol-consuming rats [74]. Akbarzadeh et al. also explored the cytotoxic activity of melatonin alone or in combination with photodynamic irradiation on HUVEC umbilical cells and SKOV3 ovarian cancer cell line. A remarkable increase in the levels of reactive oxygen species generation, apoptosis–necrosis rate, and heat shock protein 70 expression was reported in both cell lines in response to the combination of melatonin and photodynamic therapy. This can highlight the melatonin as an enhancing agent for the apoptosis and efficacy of laser therapy in ovarian cancer cells [76]. In another recent study, ZemŁA et al. have explored the effectiveness of using melatonin with the anticancer drug, cisplatin on SK-OV-3, IOSE 364, and OVCAR-3 ovarian cancer cell lines. This study demonstrated that melatonin at certain concentration showed synergistic effect with cisplatin. Moreover, this synergism found to be independent of membrane melatonin receptor MTI [77]. Ataei et al. have explored the activity of melatonin as inhibitor for the Cadmium-induced proliferation in SK-OV-3 and OVCAR-3 cell lines. While cadmium showed proliferation enhancement, melatonin showed inhibition of this cadmium-induced proliferation. Furthermore, melatonin inhibited the cadmium-induced effect on estrogen receptor α expression in SK-OV-3 and OVCAR-3 cells [75]. A study has demonstrated the effect of melatonin in ovarian cancer cells (OVCAR-429 and PA-1). It repressed cell growth and downregulated CDK2 and 4 [78]. Interestingly, using long-term treatment of melatonin in an in vivo model of ovarian carcinoma (OC), exhibited high potency of melatonin in regulating different signaling pathways associated with OC [79].
3.6. Colorectal Cancer
Colorectal cancer is a challenging cancer, with a high expected incidence in elderly people. Its signs and symptoms depend on the anatomical location, tumor progression, and cancer stage [80][81][82] However, 60% of cases can be monitored with therapies [83]. Melatonin has been used as an anticancer therapy in colorectal cancer [84][85]. Wang et al. have investigated the effect of combining melatonin with ionizing radiation on HCT 116 human colorectal cancer cell line in vitro and in vivo Melatonin inhibited proliferation, cell migration, and colony formation in HCT 116 following ionizing radiation. This increase in radiosensitivity of the cells was in association with cell cycle arrest in the phase G2/M, activation of caspas-related apoptosis, and decrease in the expression of proteins involved in break repair. In vivo, cell growth of the xenografted tumor was significantly inhibited after treatment with melatonin and ionizing radiation as compared to each agent alone, hence, higher tumor suppression rate suggesting melatonin sensitizing the colorectal cancer cells in cancer radiotherapy [85]. In an attempt to explore apoptosis activity of melatonin, Wei et al. have investigated the mechanism of melatonin-induced apoptosis in LoVo colorectal cancer cell line. It was found that melatonin inhibited proliferation and promoted apoptosis in LoVo cells. It was observed that the melatonin induced apoptosis via nuclear import and dephosphorylation of histone deacetylase 4 (HDAC4), as well as reduced the expression of Bcl-2 [84]. In another study, Yun et al. have explored the apoptic and the pro-oxidant effect of melatonin in wild type human colorectal cancer cell line (SNU-C5/WT). It was found that melatonin increased the production of superoxide via decreasing the levels of PTEN-induced kinase 1 (PINK1) and cellular prion protein (PrPC). This induces endoplasmic reticulum stress and apoptosis. The results of this study have shed the light on a promising targeting strategy in colorectal cancer [86]. In the same line, Lee et al. have investigated the PrPC level in oxaliplatin-resistant colorectal cancer (SNU-C5/Oxal-R). Significantly increased levels of PrPC was found in SNU-C5/Oxal-R as compared with SNU-C5/WT colorectal cancer. Interestingly, co-administration of melatonin with oxaliplatin downregulated the PrPC expression and increased the superoxide production. Moreover, apoptosis and endoplasmic reticulum stress were remarkably increased in SNU-C5/Oxal-R following co-administration of melatonin with oxaliplatin suggesting the role of as a key protein in resistance to oxaliplatin in SNU-C5/Oxal-R [87]. Antitumor activity of melatonin was also reported in human colorectal cancer cells (HCT116). Melatonin amplified apoptosis action, autophagy, and senescence in cancer cells [88]. Besides, it was able to prevent cell migration in RKO colon cancer cells via suppression of ROCK expression [89].
3.7. Oral Cancer
Oral cancer is a highly aggressive cancer with a high mortality rate worldwide [90]. Chemotherapy showed beneficial activity for survival in local oral cancer [91]. Liu et al. have investigated the effect of melatonin on SCC9, SCC25, Cal27, Tca8113, FaDu, and hNOKs oral cancer cells. It was found that the apoptosis resistance and proliferation were impaired upon treatment with melatonin. This effect was due to inactivation of ROS-dependent Akt signaling, downregulation of Bcl-2, PCNA, and cyclin D1. Melatonin also decreased invasion and migration of oral cancer cells [92]. Yeh et al. have explored the antimetastatic activity of melatonin in OECM-1 and HSC-3 oral cancer cell lines. Their results demonstrated that melatonin hampered the migration of OECM-1 and HSC-3 cells; in addition, it decreased the activity of MMP-9 enzyme, as well as its expression of mRNA and protein. Moreover melatonin showed a suppression effect on the phosphorylation of the ERK1/2 signaling pathway that decreased the gene transcription of MMP-9 [93]. Additionally, Yang et al. have evaluated the action of melatonin on oral cancer patient-derived tumor xenograft as a model and in oral squamous cell carcinoma. They examined the effect of overexpressing of histone lysine-specific demethylase (LSD1). Melatonin significantly suppressed the cell proliferation of oral squamous cell carcinoma in a time- and dose-dependent manner. The results suggested that proliferation suppression was associated with melatonin-induced inhibition of histone lysine-specific demethylase in oral cancer in vitro and in vivo [94]. In a recent study, Hunsaker et al. have evaluated the effect of melatonin on the microRNA content in the extracellular vesicles in different oral cancer cell lines including CAL27, SCC25, and SCC9. The results showed differential effect of melatonin on specific microRNAs in the three oral cancer cell lines highlighting the importance of evaluation of microRNA when studying the anti-oral cancer activity of melatonin [95]. Another study has shown the effect of melatonin on suppressing molecular proteins associated with angiogenesis and metastasis in oral carcinoma cells [28]. Besides, antiapoptotic activity of melatonin was reported in VCR-resistant oral cancer cells [96].
3.8. Liver Cancer
Liver cancer is the fourth leading cause of cancer death globally in 2018 [57]. Several studies have reported the efficiency of melatonin against hepatocarcinoma cells [97][33]. Ordoñez et al. have evaluated the role of melatonin in ceramides metabolism and autophagy in HepG2 cells, human liver cancer cell line. Melatonin promoted autophagy in HepG2 cells via JNK phosphorylation which is characterized by an increase in p62 degradation, Beclin-1 expression, and colocalization of LAMP-2 and LC3II that lead to decreased cell viability. Furthermore, melatonin increased the ceramides levels through acid sphingomyelinase (ASMase) stimulation and de novo synthesis indicating. Given the crucial role of ceramides in regulating the autophagy, it is indicated the effect of melatonin on autophagy and apoptosis through affecting the ceramides metabolism [98]. Carbajo-Pescador et al. have investigated the anti-angiogenic activity of melatonin in HepG2. It was found that melatonin decreased the levels of VEGF, the expression of HIF-1α protein under hypoxic conditions. Furthermore, melatonin inhibited the hypoxia-induced increase in phospho-STAT3, CBP/p300, and HIF-1α and inhibited their physical interaction, suggesting that melatonin exhibited its anti-angiogenic effect by interfering with VEGF transcriptional activation through HIF-1α and STAT3 [97]. Cheng et al. have evaluated the effect of melatonin on the exosome derived from hepatocarcinoma cells and the expression of inflammatory factors. Melatonin reduced the expression of programmed death ligand 1 on macrophages. Furthermore, melatonin inhibited the high expression of the inflammatory cytokines; TNFα, IL-10, IL-6, and IL-1β in macrophages. It was found that exosomes derived from melatonin treated hepatocarcinoma cells can change the immunosuppression state via STAT3 axis in macrophages, suggesting the role of melatonin in manipulating the immunosuppressive state in hepatocarcinoma cells [99]. Besides, melatonin has reduced the expression of HIF-1α, VEGF, and suppressed cell proliferation in hepatocarcinoma cells [100]. In addition, Human hepatoma cell apoptosis has been induced by melatonin via downregulation of COX-2 [101].
3.9. Renal Cancer
Several studies have focused on the role of melatonin as an anticancer in renal cancer [102][103]. Abraham et al. have explored whether melatonin can prevent Methotrexate-induced renal damage in rats. The results revealed that the rats which were treated with melatonin prior to methotrexate treatment showed a reduction in methotrexate-induced renal damage biochemically and histologically. Moreover, pretreatment of melatonin showed a reduction in Methotrexte-induced oxidative stress and perturbation in the antioxidant enzymes, indicating the beneficial role of melatonin in decreasing the Methotrexate-induced side effects in renal cancer cells and tissues [104]. Park et al. have investigated the mechanism underpinning melatonin effect on renal cancer Caki cells. It was shown that melatonin promoted apoptosis; it elevated the proapoptic protein Bcl-2-interacting mediator of cell death (Bim). Melatonin increased the mRNA expression of Bim through increasing the expression and transcriptional activity of E2F1 and Sp1, suggesting that melatonin promotes apoptosis in renal cancer Caki cells via increasing Bim expression [105]. Recently, Lin et al. have studied the impact of melatonin on the migration and invasion of Caki-1 and Achn renal cancer cell lines. Melatonin inhibited migration and invasion of these cells. Furthermore, melatonin decreased MMP-9 by decreasing p52- and p65-DNA-binding activities. In addition, ERK1/2 and JNK1/2 signaling pathways were implicated in the melatonin regulatory effect on cell motility and MMP-9 transactivation, indicating the impact of melatonin on motility and metastasis of renal cancer cells [106]. Table 2 summarizes the anticancer effect of melatonin on different cancer types with the mechanisms of action.
Table 2. Anticancer activities of melatonin against different cancer types.
| Cancer Type | Study Model | Dose of Melatonin | Main Effects of Melatonin and Outcomes | Reference |
|---|---|---|---|---|
| Gastric cancer | AGS and SGC-7901 cell lines mice |
1 mΜ, 2 mΜ, 3 mΜ melatonin 50 mg/kg melatonin |
inhibited cell proliferation via the activation of the IRE/JNK/Beclin1 signaling induced the expression of apoptotic and autophagy-related proteins |
[107] |
| SGC7901 cell line | 10−4 M melatonin | affected the expression of differentiation relevant factors; the gene expression of endocan was significantly increased and the activity of lactate dehydrogenase and phosphatase was downregulated | [108] | |
| SGC7901 and BGC823 cell lines | 10−4 M melatonin | decreased the motility and migration distance, remodeled cells tight junctions, and increased cells adhesion | [109] | |
| AGS and MGC803 human gastric cell lines | 3 mM melatonin | induced apoptosis by upregulating the apoptosis related proteins; Caspase 3, Caspase 9, and downregulating the phosphorylation and expression of upstream regulators MDM2 and AKT | [110] | |
| SGC7901 gastric cancer cells | 2 mM melatonin | inhibited migration, reduced viability, and induced apoptosis upregulated the expression of phosphorylated (p) p38 and c Jun N terminal kinase (p JNK) protein, and downregulated the expression of nucleic p65 |
[111] | |
| Mice Murine foregastric carcinoma (MFC) cells |
0, 25, 50 and 100 mg/kg melatonin 0, 2, 4, 6, 8 and 10 mM melatonin |
inhibited cells proliferation and decreased the tumor volume increased IL-2, IL-10, and IFN-γ expression decreased IL-6 level |
[112] | |
| Glioblastoma | Glioblastoma cell lines (U251 and T98G) | 0.1–1000 μM melatonin | Reduced cell viability and self-renewal of glioblastoma cells through blocking EZH2-NOTCH1 signaling axis. | [52] |
| U87 MG and A172 cell lines | 1 mM melatonin | induced autophagy increased the levels of LC3 II, and Beclin 1 upregulation of Bcl-2, the key initiator of autophagy enhanced the apoptosis in glioblastoma cells |
[53] | |
| U251 and U87 glioblastoma cells | 1 nM, 1 mM melatonin | blocked the expression of HIF-1α protein and inhibited the expression of vascular endothelial growth factor and matrix metalloproteinase 2 (MMP-2) under hypoxia | [54] | |
| Human normal neural stem cells hNSC.100 | 1 μM, 100 μM, 1 mM melatonin | inhibited the proliferation of glioblastoma initiating cells, decreased the clonogenic and self-renewal ability, and downregulated stem cell markers including the transcription factors sox2 oct3/4, nanog, and the transmembrane glycoprotein CD133 decreases the expression levels of de mRNA of these markers |
[55] | |
| Prostate cancer | Xenografted LNCaP in mice | 1 mg/Kg melatonin | density reduction in the xenograft micro-vessels (lower angiogenesis), and decreased the growth rate downregulated the Ki67 expression, increased the HIF-1α expression, and enhanced phosphorylation of Akt |
[31] |
| Prostate cancer cell line PC-3 cells | 1 mM melatonin | upregulated miRNA3195 and miRNA 374b under hypoxia decreased the mRNA expression of angiogenesis related genes including HIF-1α, HIF-2α and VEGF at mRNA level under hypoxia | [65] | |
| LNCaP and PC-3 prostate cancer cell lines | 1 mM melatonin | increased cell toxicity caused by hrTNF-alpha and NF-related apoptosis-inducing ligand (TRAIL) without affecting the action of docetaxel, doxorubicin, or etoposide induced phenotypic changes, and neuroendocrine differentiation | [29] | |
| Lung cancer | CL1-5 and A549cell lines | 0.1, 0.3, and 1 mM melatonin | reduced the expression of CD133 in lung cancer cells inhibited PLC, β-catenin, ERK/p38, and Twist signaling pathways to suppress lung cancer stemness |
[67] |
| CL1-0, CL1-5 and A549 cell lines male SCID mice |
0.1, 0.3, or 1 mM melatonin | reduced the lung cancer metastasis reversed the phenotype of epithelial–mesenchymal through twist inhibited Twist/Twist1 expression via MT1 receptor, p38/ERK PLC, and β-catenin signaling cascades |
[113] | |
| SK-LU-1 cell line with PBMC | 1 nm, 1 μm and 1 mM melatonin | increased apoptosis and oxidative stress via reduction in GSH, and increased cell cycle arrest | [114] | |
| Ovarian cancer | SKOV3 ovarian cancer cells | 3.4 mM melatonin | inhibited proliferation decreased the expression of the proliferation marker Ki67 reduced the ZEB1, ZEB2, vimentin, and snail expression increased E-cadherin decreased the expression of matrix metalloproteinase 9 (MMP9) |
[115] |
| OVCAR-429 and PA-1 cell lines | 0.4, 0.6, and 0.8 mM melatonin | downregulated CDK 2 and 4 which lead to accumulation of OVCAR-429 and PA-1 cells the G1 phase | [78] | |
| Rats | 200 μg/100 g bw/day | decreased the expression levels of proteins involved in important metabolic processes which are associated with energy generation, mitochondrial processes, antigen presenting and processing, hypoxia, endoplasmic reticulum stress, and cancer-associated proteoglycans overexpression of fatty acids binding proteins, ATP synthase subunit β, and heat shock protein |
[79] | |
| Colorectal cancer | HCT116 cell line (p53 wild type) | 1, 10, 100 μM melatonin | decreased plasma MT1, and increased the nuclear receptor, RORα induced apoptosis and autophagic process decreased cells population in S-phase decreased Trichostatin A-associated cardiotoxicity via inhibition of A- and E-type cyclins, and upregulation of p16 and p-p21 expression promoted G1 phase arrest |
[88] |
| RKO cell line | 1, 2, and 3 mM melatonin | downregulated the levels of Rho-associated protein kinase 2 (ROCK2), p-myosin light chains (p-MLC), and phospho (p)-myosin phosphatase targeting subunit 1 (p-MYPT1) expression increased occluding and ZO-1 expression decreased the levels of p38 phosphorylation supp-ressed the migration of RKO cells |
[89] | |
| Oral cancer | SCC9 and SCC25 cells | 1 mM melatonin | decreased cell viability in both cell lines inhibited the expression of the genes VEGF and HIF-1α under hypoxia and the expression of the gene ROCK-1 in SCC9 cells |
[28] |
| SAS and SCC9 oral cancer cell lines Vincristine (VCR)-resistant oral cancer cells; SASV32, SASV16, SCC9V16, and SCC9V32. |
0.5–2 mM melatonin. | promoted the autophagy and the apoptosis of VCR-resistant oral cancer cells via p38, AKT, and c-Jun N-terminal kinase (JNK) inhibited ATP-binding cassette B1 and 4 induced apoptosis and decreased the drug resistance in VCR-resistant oral cancer cells via increasing the expression of microRNAs |
[96] | |
| Liver cancer | HepG2 hepatocarcinoma cell line | 1 mM melatonin | decreased the cell viability and downregulated the expression of proangiogenic proteins VEGF and HIF-1α under hypoxia and in normal state reduced the cell migration and invasion |
[100] |
| HepG2 hepatocarcinoma cell line | 10−9, 10−7, 10−5 and 10−3 mol/L melatonin | enhanced apoptosis in HepG2 under ER stress via selective blocking of activating transcription factor 6 (ATF-6) inhibition of cyclooxygenase-2 (COX-2) expression, and decreasing Bcl-2/Bax ratio |
[101] | |
| Renal cancer | A498, 786-O, Achn, Caki-1, and Caki-2 cells. Mice |
0.5, 1, and 2 mM melatonin 200 mg/kg melatonin |
modulated ADAMTS1 independently of the MT1 receptor, affecting invasion and growth ability induced microRNA -181d and microRNA -let-7f targeting the non-3′-UTR and 3’-UTR of ADAMTS1 to inhibit its expression and reduce the invasive in renal cancer cells |
[116] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules26092506
References
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84.
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681.
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479.
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24.
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180.
- Pourhanifeh, M.H.; Mahdavinia, M.; Reiter, R.J.; Asemi, Z. Potential use of melatonin in skin cancer treatment: A review of current biological evidence. J. Cell. Physiol. 2019, 234, 12142–12148.
- Tan, D.-X.; Reiter, R.J. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 44–66.
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725.
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383.
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin receptors: Distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 2017, 222, 2921–2939.
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.; Cardinali, D.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838.
- Mendes, C.; Gomes, G.; Belpiede, L.T.; do Carmo Buonfiglio, D.; Motta-Teixeira, L.C.; Amaral, F.G.; Cipolla-Neto, J. The effects of melatonin daily supplementation to aged rats on the ability to withstand cold, thermoregulation and body weight. Life Sci. 2021, 265, 118769.
- Luo, J.; Zhang, Z.; Sun, H.; Song, J.; Chen, X.; Huang, J.; Lin, X.; Zhou, R. Effect of melatonin on T/B cell activation and immune regulation in pinealectomy mice. Life Sci. 2020, 242, 117191.
- He, F.; Chou, C.J.; Scheiner, M.; Poeta, E.; Yuan Chen, N.; Gunesch, S.; Hoffmann, M.; Sotriffer, C.; Monti, B.; Maurice, T.; et al. Melatonin- and Ferulic Acid-Based HDAC6 Selective Inhibitors Exhibit Pronounced Immunomodulatory Effects In Vitro and Neuroprotective Effects in a Pharmacological Alzheimer’s Disease Mouse Model. J. Med. Chem. 2021, 64, 3794–3812.
- Chen, W.; Chen, X.; Chen, A.C.; Shi, Q.; Pan, G.; Pei, M.; Yang, H.; Liu, T.; He, F. Melatonin restores the osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells by preserving SIRT1-mediated intracellular antioxidant properties. Free Radic. Biol. Med. 2020, 146, 92–106.
- Zhou, Y.; Wang, C.; Si, J.; Wang, B.; Zhang, D.; Ding, D.; Zhang, J.; Wang, H. Melatonin up-regulates bone marrow mesenchymal stem cells osteogenic action but suppresses their mediated osteoclastogenesis via MT2-inactivated NF-κB pathway. Br. J. Pharmacol. 2020, 177, 2106–2122.
- Bazyar, H.; Javid, A.Z.; Behbahani, H.B.; Moradi, F.; Poode, B.M.; Amiri, P. Consumption of melatonin supplement improves cardiovascular disease risk factors and anthropometric indices in type 2 diabetes mellitus patients: A double-blind, randomized, placebo-controlled trial. Trials 2021, 22, 1–10.
- Arendt, J.; Skene, D.J. Melatonin as a chronobiotic. Sleep Med. Rev. 2005, 9, 25–39.
- Ali, T.; Rahman, S.U.; Hao, Q.; Li, W.; Liu, Z.; Ali Shah, F.; Murtaza, I.; Zhang, Z.; Yang, X.; Liu, G.; et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020, 69, e12667.
- Al-Zaqri, N.; Pooventhiran, T.; Alsalme, A.; Warad, I.; John, A.M.; Thomas, R. Structural and physico-chemical evaluation of melatonin and its solution-state excited properties, with emphasis on its binding with novel coronavirus proteins. J. Mol. Liq. 2020, 318, 114082.
- Zhang, S.; Qi, Y.; Zhang, H.; He, W.; Zhou, Q.; Gui, S.; Wang, Y. Melatonin inhibits cell growth and migration, but promotes apoptosis in gastric cancer cell line, SGC7901. Biotech. Histochem. 2013, 88, 281–289.
- Wang, R.X.; Liu, H.; Xu, L.; Zhang, H.; Zhou, R.X. Involvement of nuclear receptor RZR/RORγ in melatonin-induced HIF-1α inactivation in SGC-7901 human gastric cancer cells. Oncol. Rep. 2015, 34, 2541–2546.
- Lissoni, P.; Barni, S.; Crispino, S.; Tancini, G.; Fraschini, F. Endocrine and immune effects of melatonin therapy in metastatic cancer patients. Eur. J. Cancer Clin. Oncol. 1989, 25, 789–795.
- Alvarez-García, V.; González, A.; Alonso-González, C.; Martínez-Campa, C.; Cos, S. Regulation of vascular endothelial growth factor by melatonin in human breast cancer cells. J. Pineal Res. 2013, 54, 373–380.
- Cos, S.; Blask, D.E. Melatonin modulates growth factor activity in MCF-7 human breast cancer cells. J. Pineal Res. 1994, 17, 25–32.
- Proietti, S.; Cucina, A.; Dobrowolny, G.; D’Anselmi, F.; Dinicola, S.; Masiello, M.G.; Pasqualato, A.; Palombo, A.; Morini, V.; Reiter, R.J.; et al. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J. Pineal Res. 2014, 57, 120–129.
- Cutando, A.; López-Valverde, A.; De Vicente, J.; Gimenez, J.L.; Carcía, I.A.; De Diego, R.G. Action of melatonin on squamous cell carcinoma and other tumors of the oral cavity (Review). Oncol. Lett. 2014, 7, 923–926.
- Goncalves Ndo, N.; Rodrigues, R.V.; Jardim-Perassi, B.V.; Moschetta, M.G.; Lopes, J.R.; Colombo, J.; Zuccari, D.A. Molecular markers of angiogenesis and metastasis in lines of oral carcinoma after treatment with melatonin. Anticancer Agents Med. Chem. 2014, 14, 1302–1311.
- Rodriguez-Garcia, A.; Mayo, J.C.; Hevia, D.; Quiros-Gonzalez, I.; Navarro, M.; Sainz, R.M. Phenotypic changes caused by melatonin increased sensitivity of prostate cancer cells to cytokine-induced apoptosis. J. Pineal Res. 2013, 54, 33–45.
- Shiu, S.Y.; Leung, W.Y.; Tam, C.W.; Liu, V.W.; Yao, K.M. Melatonin MT1 receptor-induced transcriptional up-regulation of p27(Kip1) in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor kappa B (NF-κB): Potential implications on prostate cancer chemoprevention and therapy. J. Pineal Res. 2013, 54, 69–79.
- Paroni, R.; Terraneo, L.; Bonomini, F.; Finati, E.; Virgili, E.; Bianciardi, P.; Favero, G.; Fraschini, F.; Reiter, R.J.; Rezzani, R.; et al. Antitumour activity of melatonin in a mouse model of human prostate cancer: Relationship with hypoxia signalling. J. Pineal Res. 2014, 57, 43–52.
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38.
- Martín-Renedo, J.; Mauriz, J.L.; Jorquera, F.; Ruiz-Andrés, O.; González, P.; González-Gallego, J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J. Pineal Res. 2008, 45, 532–540.
- Korkmaz, A.; Tamura, H.; Manchester, L.C.; Ogden, G.B.; Tan, D.X.; Reiter, R.J. Combination of melatonin and a peroxisome proliferator-activated receptor-gamma agonist induces apoptosis in a breast cancer cell line. J. Pineal Res. 2009, 46, 115–116.
- Wang, R.X.; Liu, H.; Xu, L.; Zhang, H.; Zhou, R.X. Melatonin downregulates nuclear receptor RZR/RORγ expression causing growth-inhibitory and anti-angiogenesis activity in human gastric cancer cells in vitro and in vivo. Oncol. Lett. 2016, 12, 897–903.
- Song, J.; Ma, S.-J.; Luo, J.-H.; Zhang, H.; Wang, R.-X.; Liu, H.; Li, L.; Zhang, Z.-G.; Zhou, R.-X. Melatonin induces the apoptosis and inhibits the proliferation of human gastric cancer cells via blockade of the AKT/MDM2 pathway. Oncol. Rep. 2018, 39, 1975–1983.
- Crocetti, E.; Trama, A.; Stiller, C.; Caldarella, A.; Soffietti, R.; Jaal, J.; Weber, D.C.; Ricardi, U.; Slowinski, J.; Brandes, A. Epidemiology of glial and non-glial brain tumours in Europe. Eur. J. Cancer 2012, 48, 1532–1542.
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017, 19 (Suppl. 5), v1–v88.
- Wrensch, M.; Minn, Y.; Chew, T.; Bondy, M.; Berger, M.S. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro Oncol. 2002, 4, 278–299.
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760.
- Chen, J.; Li, Y.; Yu, T.-S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526.
- Gilbert, C.A.; Ross, A.H. Cancer stem cells: Cell culture, markers, and targets for new therapies. J. Cell. Biochem. 2009, 108, 1031–1038.
- Dirks, P.B. Brain tumor stem cells: The cancer stem cell hypothesis writ large. Mol. Oncol. 2010, 4, 420–430.
- Liebelt, B.D.; Shingu, T.; Zhou, X.; Ren, J.; Shin, S.A.; Hu, J. Glioma Stem Cells: Signaling, Microenvironment, and Therapy. Stem Cells Int. 2016, 2016, 7849890.
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111.
- Chen, X.; Hao, A.; Li, X.; Du, Z.; Li, H.; Wang, H.; Yang, H.; Fang, Z. Melatonin inhibits tumorigenicity of glioblastoma stem-like cells via the AKT-EZH2-STAT3 signaling axis. J. Pineal Res. 2016, 61, 208–217.
- Maitra, S.; Bhattacharya, D.; Das, S.; Bhattacharya, S. Melatonin and its anti-glioma functions: A comprehensive review. Rev. Neurosci. 2019, 30, 527–541.
- Neamati, F.; Asemi, Z. The effects of melatonin on signaling pathways and molecules involved in glioma. Fundam. Clin. Pharmacol. 2020, 34, 192–199.
- Sung, G.J.; Kim, S.H.; Kwak, S.; Park, S.H.; Song, J.H.; Jung, J.H.; Kim, H.; Choi, K.C. Inhibition of TFEB oligomerization by co-treatment of melatonin with vorinostat promotes the therapeutic sensitivity in glioblastoma and glioma stem cells. J. Pineal Res. 2019, 66, e12556.
- Lai, S.W.; Liu, Y.S.; Lu, D.Y.; Tsai, C.F. Melatonin Modulates the Microenvironment of Glioblastoma Multiforme by Targeting Sirtuin 1. Nutrients 2019, 11, 1343.
- Fernandez-Gil, B.; Schiappareli, P.; Vazquez-Ramos, C.; Sarabia-Estrada, R.; Escames, G.; Quiñones-Hinojosa, A. CADD-28. Melatonin: Targeting the cell’s powerhouse to fight glioblastoma. Neuro Oncol. 2018, 20, vi280–vi281.
- Zheng, X.; Pang, B.; Gu, G.; Gao, T.; Zhang, R.; Pang, Q.; Liu, Q. Melatonin Inhibits Glioblastoma Stem-like cells through Suppression of EZH2-NOTCH1 Signaling Axis. Int. J. Biol. Sci. 2017, 13, 245–253.
- Martín, V.; Sanchez-Sanchez, A.M.; Puente-Moncada, N.; Gomez-Lobo, M.; Alvarez-Vega, M.A.; Antolín, I.; Rodriguez, C. Involvement of autophagy in melatonin-induced cytotoxicity in glioma-initiating cells. J. Pineal Res. 2014, 57, 308–316.
- Zhang, Y.; Liu, Q.; Wang, F.; Ling, E.A.; Liu, S.; Wang, L.; Yang, Y.; Yao, L.; Chen, X.; Wang, F. Melatonin antagonizes hypoxia-mediated glioblastoma cell migration and invasion via inhibition of HIF-1α. J. Pineal Res. 2013, 55, 121–130.
- Zhou, N.; Wei, Z.X.; Qi, Z.X. Inhibition of autophagy triggers melatonin-induced apoptosis in glioblastoma cells. BMC Neurosci. 2019, 20, 1–12.
- 2019. G. C. O. C. t. d. t. h. g. i. f. t. h. A. J. Available online: (accessed on 25 February 2021).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Mayo, J.C.; Hevia, D.; Quiros-Gonzalez, I.; Rodriguez-Garcia, A.; Gonzalez-Menendez, P.; Cepas, V.; Gonzalez-Pola, I.; Sainz, R.M. IGFBP3 and MAPK/ERK signaling mediates melatonin-induced antitumor activity in prostate cancer. J. Pineal Res. 2017, 62, e12373.
- Tai, S.Y.; Huang, S.P.; Bao, B.Y.; Wu, M.T. Urinary melatonin-sulfate/cortisol ratio and the presence of prostate cancer: A case-control study. Sci. Rep. 2016, 6, 29606.
- Sigurdardottir, L.G.; Markt, S.C.; Rider, J.R.; Haneuse, S.; Fall, K.; Schernhammer, E.S.; Tamimi, R.M.; Flynn-Evans, E.; Batista, J.L.; Launer, L.; et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur. Urol. 2015, 67, 191–194.
- Wang, S.-W.; Tai, H.-C.; Tang, C.-H.; Lin, L.-W.; Lin, T.-H.; Chang, A.-C.; Chen, P.-C.; Chen, Y.-H.; Wang, P.-C.; Lai, Y.-W.; et al. Melatonin impedes prostate cancer metastasis by suppressing MMP-13 expression. J. Cell. Physiol. 2020, 236, 3979–3990.
- Zharinov, G.M.; Bogomolov, O.A.; Chepurnaya, I.V.; Neklasova, N.Y.; Anisimov, V.N. Melatonin increases overall survival of prostate cancer patients with poor prognosis after combined hormone radiation treatment. Oncotarget 2020, 11, 3723–3729.
- Liu, V.W.S.; Yau, W.L.; Tam, C.W.; Yao, K.M.; Shiu, S.Y.W. Melatonin Inhibits Androgen Receptor Splice Variant-7 (AR-V7)-Induced Nuclear Factor-Kappa B (NF-κB) Activation and NF-κB Activator-Induced AR-V7 Expression in Prostate Cancer Cells: Potential Implications for the Use of Melatonin in Castration-Resistant Prostate Cancer (CRPC) Therapy. Int. J. Mol. Sci. 2017, 18, 1130.
- Tamarindo, G.H.; Ribeiro, D.L.; Gobbo, M.G.; Guerra, L.H.A.; Rahal, P.; Taboga, S.R.; Gadelha, F.R.; Góes, R.M. Melatonin and Docosahexaenoic Acid Decrease Proliferation of PNT1A Prostate Benign Cells via Modulation of Mitochondrial Bioenergetics and ROS Production. Oxidative Med. Cell. Longev. 2019, 2019, 5080798.
- Sohn, E.J.; Won, G.; Lee, J.; Lee, S. Kim Sh. Upregulation of miRNA3195 and miRNA374b Mediates the Anti-Angiogenic Properties of Melatonin in Hypoxic PC-3 Prostate Cancer Cells. J. Cancer 2015, 6, 19.
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66.
- Yang, Y.-C.; Chiou, P.-C.; Chen, P.-C.; Liu, P.-Y.; Huang, W.-C.; Chao, C.-C.; Tang, C.-H. Melatonin reduces lung cancer stemness through inhibiting of PLC, ERK, p38, β-catenin, and Twist pathways. Environ. Toxicol. 2019, 34, 203–209.
- 2018. A. C. S. C. f. f.; American Cancer Society, N.Y. Available online: (accessed on 25 February 2021).
- Ma, Z.; Liu, D.; Di, S.; Zhang, Z.; Li, W.; Zhang, J.; Xu, L.; Guo, K.; Zhu, Y.; Li, X.; et al. Histone deacetylase 9 downregulation decreases tumor growth and promotes apoptosis in non-small cell lung cancer after melatonin treatment. J. Pineal Res. 2019, 67, e12587.
- Yun, M.; Kim, E.O.; Lee, D.; Kim, J.H.; Kim, J.; Lee, H.; Lee, J.; Kim, S.H. Melatonin Sensitizes H1975 Non-Small-Cell Lung Cancer Cells Harboring a T790M-Targeted Epidermal Growth Factor Receptor Mutation to the Tyrosine Kinase Inhibitor Gefitinib. Cell. Physiol. Biochem. 2014, 34, 865–872.
- Plaimee, P.; Weerapreeyakul, N.; Barusrux, S.; Johns, N.P. Melatonin potentiates cisplatin-induced apoptosis and cell cycle arrest in human lung adenocarcinoma cells. Cell Prolif. 2015, 48, 67–77.
- Zhou, Q.; Gui, S.; Zhou, Q.; Wang, Y. Melatonin inhibits the migration of human lung adenocarcinoma A549 cell lines involving JNK/MAPK pathway. PLoS ONE 2014, 9, e101132.
- Cannistra, S.A. Cancer of the ovary. N. Engl. J. Med. 2004, 351, 2519–2529.
- Chuffa, L.G.A.; Fioruci-Fontanelli, B.A.; Mendes, L.O.; Ferreira Seiva, F.R.; Martinez, M.; Fávaro, W.J.; Domeniconi, R.F.; Pinheiro, P.F.F.; Delazari dos Santos, L.; Martinez, F.E. Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 2015, 15, 34.
- Ataei, N.; Aghaei, M.; Panjehpour, M. The protective role of melatonin in cadmium-induced proliferation of ovarian cancer cells. Res. Pharm. Sci. 2018, 13, 159–167.
- Akbarzadeh, M.; Nouri, M.; Banekohal, M.V.; Cheraghi, O.; Tajalli, H.; Movassaghpour, A.; Soltani, S.; Cheraghi, H.; Feizy, N.; Montazersaheb, S.; et al. Effects of combination of melatonin and laser irradiation on ovarian cancer cells and endothelial lineage viability. Lasers Med. Sci. 2016, 31, 1565–1572.
- Zemła, A.; Grzegorek, I.; Dzięgiel, P.; Jabłońska, K. Melatonin Synergizes the Chemotherapeutic Effect of Cisplatin in Ovarian Cancer Cells Independently of MT1 Melatonin Receptors. In Vivo 2017, 31, 801.
- Shen, C.-J.; Chang, C.-C.; Chen, Y.-T.; Lai, C.-S.; Hsu, Y.-C. Melatonin Suppresses the Growth of Ovarian Cancer Cell Lines (OVCAR-429 and PA-1) and Potentiates the Effect of G1 Arrest by Targeting CDKs. Int. J. Mol. Sci. 2016, 17, 176.
- Chuffa, L.G.A.; Lupi Júnior, L.A.; Seiva, F.R.F.; Martinez, M.; Domeniconi, R.F.; Pinheiro, P.F.F.; dos Santos, L.D.; Martinez, F.E. Quantitative Proteomic Profiling Reveals That Diverse Metabolic Pathways Are Influenced by Melatonin in an in Vivo Model of Ovarian Carcinoma. J. Proteome Res. 2016, 15, 3872–3882.
- Astin, M.; Griffin, T.; Neal, R.D.; Rose, P.; Hamilton, W. The diagnostic value of symptoms for colorectal cancer in primary care: A systematic review. Br. J. Gen. Pr. 2011, 61, e231–e243.
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560.
- Farooqi, A.A.; de la Roche, M.; Djamgoz, M.B.A.; Siddik, Z.H. Overview of the oncogenic signaling pathways in colorectal cancer: Mechanistic insights. Semin. Cancer Biol. 2019, 58, 65–79.
- Hegde, S.R.; Sun, W.; Lynch, J.P. Systemic and targeted therapy for advanced colon cancer. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 135–149.
- Wei, J.-Y.; Li, W.-M.; Zhou, L.-L.; Lu, Q.-N.; He, W. Melatonin induces apoptosis of colorectal cancer cells through HDAC4 nuclear import mediated by CaMKII inactivation. J. Pineal Res. 2015, 58, 429–438.
- Wang, Q.; Sun, Z.; Du, L.; Xu, C.; Wang, Y.; Yang, B.; He, N.; Wang, J.; Ji, K.; Liu, Y.; et al. Melatonin Sensitizes Human Colorectal Cancer Cells to γ-ray Ionizing Radiation In Vitro and In Vivo. Int. J. Mol. Sci. 2018, 19, 3974.
- Yun, C.W.; Kim, S.; Lee, J.H.; Lee, S.H. Melatonin promotes apoptosis of colorectal cancer cells via superoxide-mediated ER stress by inhibiting cellular prion protein expression. Anticancer Res. 2018, 38, 3951–3960.
- Lee, J.H.; Yoon, Y.M.; Han, Y.-S.; Yun, C.W.; Lee, S.H. Melatonin Promotes Apoptosis of Oxaliplatin-resistant Colorectal Cancer Cells Through Inhibition of Cellular Prion Protein. Anticancer Res. 2018, 38, 1993.
- Hong, Y.; Won, J.; Lee, Y.; Lee, S.; Park, K.; Chang, K.-T.; Hong, Y. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J. Pineal Res. 2014, 56, 264–274.
- Liu, Z.; Zou, D.; Yang, X.; Xue, X.; Zuo, L.; Zhou, Q.; Hu, R.; Wang, Y. Melatonin inhibits colon cancer RKO cell migration by downregulating Rho-associated protein kinase expression via the p38/MAPK signaling pathway. Mol. Med. Rep. 2017, 16, 9383–9392.
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108.
- Su, S.-C.; Lin, C.-W.; Liu, Y.-F.; Fan, W.-L.; Chen, M.-K.; Yu, C.-P.; Yang, W.-E.; Su, C.-W.; Chuang, C.-Y.; Li, W.-H.; et al. Exome Sequencing of Oral Squamous Cell Carcinoma Reveals Molecular Subgroups and Novel Therapeutic Opportunities. Theranostics 2017, 7, 1088–1099.
- Liu, R.; Wang, H.-l.; Deng, M.-j.; Wen, X.-j.; Mo, Y.-y.; Chen, F.-m.; Zou, C.-l.; Duan, W.-f.; Li, L.; Nie, X. Melatonin Inhibits Reactive Oxygen Species-Driven Proliferation, Epithelial-Mesenchymal Transition, and Vasculogenic Mimicry in Oral Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 3510970.
- Yeh, C.-M.; Lin, C.-W.; Yang, J.-S.; Yang, W.-E.; Su, S.-C.; Yang, S.-F. Melatonin inhibits TPA-induced oral cancer cell migration by suppressing matrix metalloproteinase-9 activation through the histone acetylation. Oncotarget 2016, 7, 21952–21967.
- Yang, C.-Y.; Lin, C.-K.; Tsao, C.-H.; Hsieh, C.-C.; Lin, G.-J.; Ma, K.-H.; Shieh, Y.-S.; Sytwu, H.-K.; Chen, Y.-W. Melatonin exerts anti-oral cancer effect via suppressing LSD1 in patient-derived tumor xenograft models. Oncotarget 2017, 8, 33756–33769.
- Hunsaker, M.; Barba, G.; Kingsley, K.; Howard, K.M. Differential MicroRNA Expression of miR-21 and miR-155 within Oral Cancer Extracellular Vesicles in Response to Melatonin. Dent. J. 2019, 7, 48.
- Hsieh, M.-J.; Lin, C.-W.; Su, S.-C.; Reiter, R.J.; Chen, A.W.-G.; Chen, M.-K.; Yang, S.-F. Effects of miR-34b/miR-892a Upregulation and Inhibition of ABCB1/ABCB4 on Melatonin-Induced Apoptosis in VCR-Resistant Oral Cancer Cells. Mol. Ther. Nucleic Acids 2020, 19, 877–889.
- Carbajo-Pescador, S.; Ordoñez, R.; Benet, M.; Jover, R.; García-Palomo, A.; Mauriz, J.L.; González-Gallego, J. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br. J. Cancer 2013, 109, 83–91.
- Ordoñez, R.; Fernández, A.; Prieto-Domínguez, N.; Martínez, L.; García-Ruiz, C.; Fernández-Checa, J.C.; Mauriz, J.L.; González-Gallego, J. Ceramide metabolism regulates autophagy and apoptotic cell death induced by melatonin in liver cancer cells. J. Pineal Res. 2015, 59, 178–189.
- Cheng, L.; Liu, J.; Liu, Q.; Liu, Y.; Fan, L.; Wang, F.; Yu, H.; Li, Y.; Bu, L.; Li, X.; et al. Exosomes from Melatonin Treated Hepatocellularcarcinoma Cells Alter the Immunosupression Status through STAT3 Pathway in Macrophages. Int. J. Biol. Sci. 2017, 13, 723–734.
- Colombo, J.; Maciel, J.M.W.; Ferreira, L.C.; Da Silva, R.F.; Zuccari, D.A.P.D.C. Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells. Oncol. Lett. 2016, 12, 231–237.
- Bu, L.-J.; Yu, H.-Q.; Fan, L.-L.; Li, X.-Q.; Wang, F.; Liu, J.-T.; Zhong, F.; Zhang, C.-J.; Wei, W.; Wang, H.; et al. Melatonin, a novel selective ATF-6 inhibitor, induces human hepatoma cell apoptosis through COX-2 downregulation. World J. Gastroenterol. 2017, 23, 986–998.
- Neri, B.; Fiorelli, C.; Moroni, F.; Nicita, G.; Paoletti, M.C.; Ponchietti, R.; Raugei, A.; Santoni, G.; Trippitelli, A.; Grechi, G. Modulation of human lymphoblastoid interferon activity by melatonin in metastatic renal cell carcinoma. A phase II study. Cancer 1994, 73, 3015–3019.
- Min, K.J.; Kim, H.S.; Park, E.J.; Kwon, T.K. Melatonin enhances thapsigargin-induced apoptosis through reactive oxygen species-mediated upregulation of CCAAT-enhancer-binding protein homologous protein in human renal cancer cells. J. Pineal Res. 2012, 53, 99–106.
- Abraham, P.; Kolli, V.K.; Rabi, S. Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem. Funct. 2010, 28, 426–433.
- Park, E.J.; Woo, S.M.; Min, K.-j.; Kwon, T.K. Transcriptional and post-translational regulation of Bim controls apoptosis in melatonin-treated human renal cancer Caki cells. J. Pineal Res. 2014, 56, 97–106.
- Lin, Y.-W.; Lee, L.-M.; Lee, W.-J.; Chu, C.-Y.; Tan, P.; Yang, Y.-C.; Chen, W.-Y.; Yang, S.-F.; Hsiao, M.; Chien, M.-H. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J. Pineal Res. 2016, 60, 277–290.
- Zheng, Y.; Tu, J.; Wang, X.; Yu, Y.; Li, J.; Jin, Y.; Wu, J. The Therapeutic Effect of Melatonin on GC by Inducing Cell Apoptosis and Autophagy Induced by Endoplasmic Reticulum Stress. Onco Targets 2019, 12, 10187–10198.
- Zhang, S.; Zuo, L.; Gui, S.; Zhou, Q.; Wei, W.; Wang, Y. Induction of cell differentiation and promotion of endocan gene expression in stomach cancer by melatonin. Mol. Biol. Rep. 2012, 39, 2843–2849.
- Wei, X.; Chen, S.; Xu, Z.; Jia, N.; Qi, Y.; Zhou, Q.; Wang, J.; Qu, L.; Zhang, S.; Wang, Y. Melatonin inhibits the migration of human gastric carcinoma cells at least in part by remodeling tight junction. J. Cell. Biochem. 2019, 120, 9781–9786.
- Song, J.; Ma, S.-J.; Luo, J.-H.; Liu, H.; Li, L.; Zhang, Z.-G.; Chen, L.-S.; Zhou, R.-X. Downregulation of AKT and MDM2, Melatonin Induces Apoptosis in AGS and MGC803 Cells. Anat. Rec. 2019, 302, 1544–1551.
- Li, W.; Wang, Z.; Chen, Y.; Wang, K.; Lu, T.; Ying, F.; Fan, M.; Li, Z.; Wu, J. Melatonin treatment induces apoptosis through regulating the nuclear factor-κB and mitogen-activated protein kinase signaling pathways in human gastric cancer SGC7901 cells. Oncol. Lett. 2017, 13, 2737–2744.
- Kai, C.R. Effect of melatonin on the expression of Th1/Th2/Th17 cytokines of gastric cancer in vitro and in vivo. Acta Anat. Sin. 2019, 50, 471–476.
- Chao, C.-C.; Chen, P.-C.; Chiou, P.-C.; Hsu, C.-J.; Liu, P.-I.; Yang, Y.-C.; Reiter, R.J.; Yang, S.-F.; Tang, C.-H. Melatonin suppresses lung cancer metastasis by inhibition of epithelial–mesenchymal transition through targeting to Twist. Clin. Sci. 2019, 133, 709–722.
- Plaimee, P.; Khamphio, M.; Weerapreeyakul, N.; Barusrux, S.; Johns, N.P. Immunomodulatory effect of melatonin in SK-LU-1 human lung adenocarcinoma cells co-cultured with peripheral blood mononuclear cells. Cell Prolif. 2014, 47, 406–415.
- Akbarzadeh, M.; Movassaghpour, A.A.; Ghanbari, H.; Kheirandish, M.; Fathi Maroufi, N.; Rahbarghazi, R.; Nouri, M.; Samadi, N. The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells. Sci. Rep. 2017, 7, 17062.
- Wen, Y.-C.; Lin, Y.-W.; Chu, C.-Y.; Yang, Y.-C.; Yang, S.-F.; Liu, Y.-F.; Hsiao, M.; Lee, W.-J.; Chien, M.-H. Melatonin-triggered post-transcriptional and post-translational modifications of ADAMTS1 coordinately retard tumorigenesis and metastasis of renal cell carcinoma. J. Pineal Res. 2020, 69, e12668.
This entry is offline, you can click here to edit this entry!
