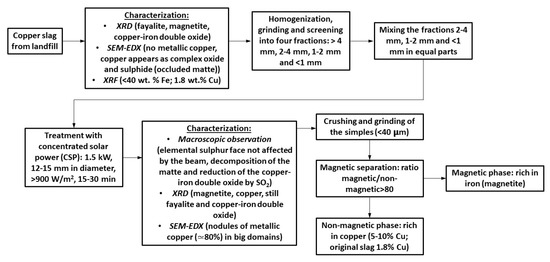
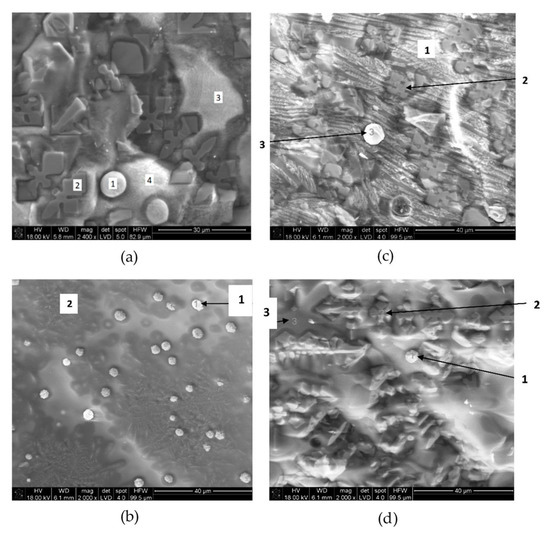
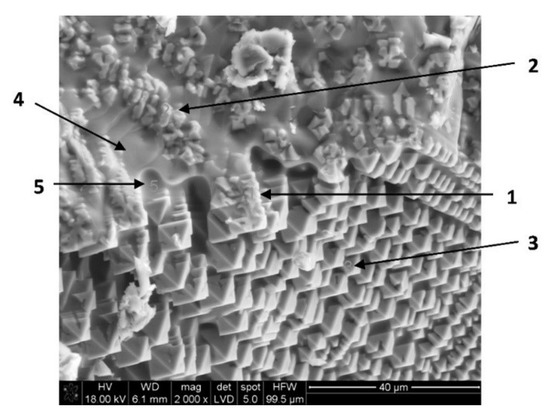
On the one hand, copper slag is nowadays a waste in copper pyrometallurgy despite the significant quantities of iron (>40 wt. %) and copper (1 to 2 wt. %). On the other hand, solar energy, when properly concentrated, offers great potential in high-temperature processes. Therefore, concentrated solar power (CSP) could be used in the treatment of copper slag to transform fayalite into magnetite and copper sulfides and oxides into copper nodules. This is the objective of this paper. The results show that fayalite was partially decomposed into magnetite and silica. Moreover, copper nodules (65–85 wt. % Cu) were identified in the treated samples, while the initial slag, analyzed by X-ray diffraction, X-ray fluorescence, and SEM-EDX, did not show the presence of metallic copper. Finally, the treated copper slag was crushed and grinded down to 40 μm, and two fractions were obtained by magnetic separation. The magnetic fraction (85%) was mainly comprised of magnetite, while the non-magnetic fraction (15%) had 5–10 wt. % Cu. Considering the experimental results, 7.5–18 kg Cu/t slag might be recovered from the slag. A preliminary economic analysis, considering the current copper price, indicates that only the recovery of copper could represent a significant economic benefit (>30 €/t slag). Therefore, CSP might be a potential candidate for the treatment of copper slag to recover copper and iron.
1. Introduction
Copper is one of the most mined metals on the Earth due to the applications of this metal in different fields. However, a lot of tailing material, currently unprocessed, is generated in the process, and provides a huge opportunity to use concentrated solar power (CSP).
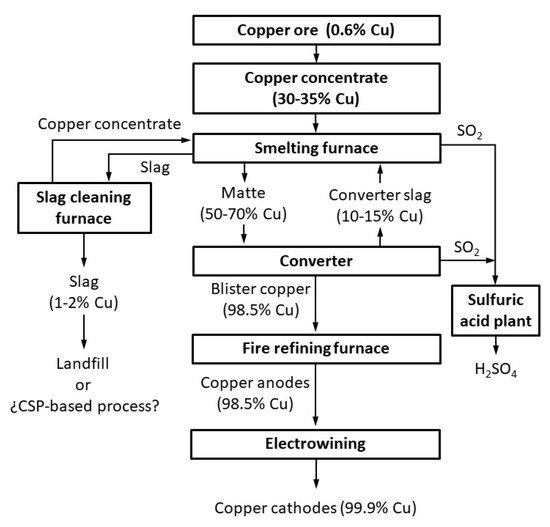
The pyrometallurgical technique is the most important to produce copper, and the smelting conversion process is the most widely used within this route [
1]. Thus, 80% of the copper is currently produced by the concentration, smelting, and refining of sulfide ores [
2], which include chalcopyrite (CuFeS2), bornite (Cu5FeS4), and chalcocite (Cu2S). Different products are generated in the fusion conversion process (
Figure 1). These include matte, which is heavy and contains most of the copper as sulfide (it is later treated in the converter to obtain blister copper, and finally metallic copper is produced after fire refining and electrowinning) and slag, which contains most of the iron as fayalite.
Two slags are produced in the smelting conversion process: first, in the smelting furnace, and second in the converter. However, these slags contain (apart from fayalite and magnetite, which are the main phases) significant quantities of copper (sulfide (matte) dragged or trapped by the slag, or oxide associated with other oxides of the slag [
1,
3]). The slags are reprocessed in the smelting furnace or in the slag cleaning furnace (electric furnaces, where carbon and pyrite are used as additives) to recover some of the copper. Finally, the final slag is disposed of in a controlled landfill when the copper concentration is approximately in the range 1 to 2 wt. % Cu.
Copper slags might be considered a residue/by-product with great potential due to both the copper (1–2 wt. % Cu) and iron (>40 wt. % [
4,
5,
6,
7,
8,
9,
10]) contents as a secondary resource for metal recovery [
11]. A total of 20 Mt of primary copper are produced worldwide, and this involves 45 Mt of slag generated in the process (2.2–3 tons of slag/ton copper [
2,
12,
13]), which would represent >20 Mt Fe and 0.5–1 Mt Cu yearly sent to controlled landfills. Therefore, copper slag represents an environmental impact [
4]: risks of heavy metals lixiviation, a visual impact, and sometimes occupation of cultivable areas. Researchers have tried to find a use for copper slags, but a massive industrial utilization has still not been found [
14]. Copper slags have been used as abrasives (polishing and cleaning) for metallic structures [
15,
16] and mainly in the building industry: concrete manufactured with copper slag [
5]; copper slags as fine particles in concrete manufacturing [
13]; copper slag as a replacement for the sand in cements [
9]; copper slag as a filler in glass–epoxy composites [
17]; and copper slag as a construction material in bituminous pavements [
18]. Research has also focused on the recovery of iron from the slag: by using coke as reductant of the copper oxide and the magnetite [
19]; by modifying the molten slag with the purpose of promoting the mineralization of recoverable mineral phases and inducing the growth of the mineral phases [
20]; by using a method based on coal, Direct Reduction Iron (DRI), and magnetic separation [
21]; by means of a process based on aluminothermic reduction [
22]; by reduction in an electric furnace with the objective of obtaining a Cu-Pb-Fe alloy [
23]; by carbothermic reduction to transform the copper slag into pig iron and glassy material [
24]; by irradiation with a microwave as a support of the carbothermal method [
25]; or by reduction with coke powders and magnetic separation [
26].
Solar energy has been widely used in materials science and metallurgy [
27]. The main types of research in the field of metallurgy carried out with concentrated solar power (CSP) are listed in
Table 1.
Concentrated solar power (CSP) has also been used in materials processing and non-metallic materials. The use of CSP in these latter fields can be found in Fernández-González et al. [
27]. The application of CSP in materials science and metallurgy has been widely investigated by our research group. The following fields can be mentioned: synthesis of calcium aluminates [
50], iron metallurgy [
46,
51,
52], the treatment of Basic Oxygen Furnace (BOF) slag [
53], the production of silicomanganese [
54], and transformations in the Ca-Si-O system [
55].
The utilization of concentrated solar power (CSP) to treat copper slag is proposed in this paper. Since most of the copper is produced in mines located in the west of the American continent (from Arizona (United States) to Chile), where high values of solar radiation are measured, the use of CSP might have great potential to recover copper and iron from slags. Figure 2 shows a flow-sheet of the process to examine Cu tailings using CSP. The main results are schematically indicated. Therefore, the aims of the research are: to transform the fayalite into magnetite, as iron could be collected from the slag as magnetite using magnetic methods; and to transform copper oxide and copper matte (the occluded matte in the slag) into metallic copper and concentrate it.
This entry is adapted from the peer-reviewed paper 10.3390/met11071032