Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Reniera is one subgenus of Haliclona sponges and has a soft texture and brownish-maroon epidermis, and its body looks like a compressed tree with simple digitate branches and spicules of various sizes and harbors a special arrangement of the flagellated chambers in the incurrent and excurrent canal systems.
- marine sponge
- Haliclona
- Reniera
- natural product
1. Introduction
Marine sponges are widely distributed across oceans and represent one of the most diverse groups of primitive multicellular aquatic animals in nature. Numerous chemical investigations have indicated that this marine creature is one of the most attractive sources of precious natural products with the potential of clinical application [1]. As one of the important marine sponges found in the Mediterranean Sea and Atlantic area, Reniera was originally assigned as one genus and later classified to be one subgenus of Haliclona [2]. Morphologically, Reniera sponge has a soft texture and brownish-maroon epidermis, and its body is soft and fragile and looks like a compressed tree with simple digitate branches and spicules of various sizes [3]. Meanwhile, this marine sponge harbors a special arrangement of the flagellated chambers in the incurrent and excurrent canal systems [3]. To the best of our knowledge, the subgenus Reniera consists of at least 16 species, including R. albescens, R. coccinea, R. cratera, R. fallaciosa, R. fascigera, R. fragilis, R. fulva, R japonica, R. lacteal, R. membrana, R. mucosa, R. porosa, R. porrecta, R. reticulata, R. sarai, and R. thomasii [4].
2. Natural Product Inventory of the Subgenus Reniera
Chemical studies of the marine sponge subgenus Reniera date back to the early 1970s. Until 2020, as many as 121 natural products had been isolated and characterized from Reniera sponges and their endozoic microbes. According to their chemical structures, these biomolecules are grouped into five types including alkaloid, terpenoid, polyketide, sterol, and cerebroside and ceramide, which are respectively introduced in detail as follows.
2.1. Alkaloids
2.1.1. 3-Alkylpyridiniums
Reniera sponge-derived 3-alkylpryridiniums are inseparable dimers or polymers with various degrees of polymerization (DP) and different lengths of alkyl chains. Usually, polymeric 3-alkylpyridinium salt (Poly-APS) possesses a broad spectrum of biological properties, including a potent inhibitory effect on acetylcholinesterase and phosphatase 2A, and cytotoxic, hemoclasis, and proarrhythmogenic activity [5][6][7][8][9][10][11][12][13]. Moreover, these natural products had been found to inhibit microfouling, and the proliferation and movement of susceptible algae and biofilm bacteria [14][15][16]. The chemical study of Reniera sp. collected from Pemba Island (Tanzania) afforded three novel cyclic 3-alkylpyridiniums named njaoaminiums A (1), B (2), and C (3) (Figure 1), of which compound 2 has weak cytotoxicity against three human tumor cell lines A549 (lung carcinoma), HT29 (colon carcinoma), and MDA-MB-231 (breast) with GI50 values of 4.1, 4.2, and 4.8 μM, respectively [17]. Two cyclic poly-APSs (4 and 5) with a respective DP of 29 and 99 were separated and characterized from the Mediterranean specimen of R. sarai [18]. One search for the chemical synthesis of poly-APS resulted in the production of three novel analogs APS8 (6), APS12-2 (7), and APS3 (8), of which compound 8 is a mixture of two polymers with DPs of 10 and 32 covalently linked N-butyl-3-butyl pyridinium units in a 9:1 ratio. Bioassay suggested that compound 6 exhibited a toxic effect on the non-small cell lung cancer (NSCLC) tumor cell line but nontoxicity against normal lung fibroblasts [9], while 7 could cause vascular smooth muscle contraction and a decrease in arterial blood pressure and 8 could block muscle-type nicotinic acetylcholine receptors (nAChRs) [19][20].
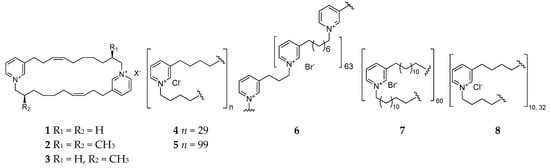
Figure 1. 3-Alkylpyridiniums 1–5 from the subgenus Reniera and their derivatives 6–8.
2.1.2. Quinolines and Isoquinolines
Bioassay fractionation of the 2-propanol crude extract of Reniera sp. collected from the Njao area (Tanzania) afforded eight new polycyclic quinolines and njaoamines A–G and I (9–16) (Figure 2); compounds 9–14 demonstrated potent cytotoxicity against human tumor cell lines colon H-T29, lung A-549, and breast MDA-MB-231 with GI50 values ranging from 1.5 to 7.2 μM; and compound 16 had cytotoxic effect on three human tumor cell lines including MDA-MB-231 (breast), HT-29 (colon), and NSLC A-549 in the micromolar range [21][22]. Chemical analysis of the similar specimen collected in Isla Grande (Mexico) afforded nine antimicrobial isoquinolines (17–25), including five monomers renierone (17), 7-methoxy-1,6-dimethyl-5,8-dihydroisoquinoline-5,8-dione (18), N-formyl-1,2-dihydrorenierone (19), O-demethylrenierone (20), and mimosamycin (21), and four dimers renieramycins A–D (22–25) [23][24]. In addition, two new polycyclic isoquinoline dimers, renieramycins E (26) and F (27), were purified from another Reniera specimen collected from Palau [25].
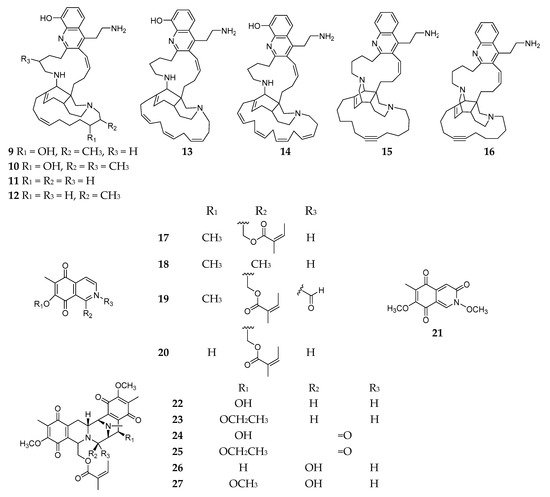
Figure 2. Quinolines and isoquinolines 9–27 from the subgenus Reniera.
2.1.3. Macrocyclic Diamines
To the best of our knowledge, all macrocyclic diamine-producing Reniera sponges were collected from the Mediterranean. Saraines A–C (28–30) (Figure 3) were obtained from the Mediterranean sponge R. sarai and exhibited a broad spectrum of biological activities, including insecticidal and acaricidal potency to the arthropoda Macrosiphum euphorbiae (Thos.), Tetranychus urticae Koch, and Aedes aegypti L.; strong inhibitory effect on Streptococcus agalactiae and AChE; and high hemolysis [26][27][28]. Chemical synthesis of compound 28 had first been achieved by Garg and coworkers in 2006 [29]. Isosaraine-1–3 (31–33) were three new hexahydro-quinolizin-2(6H)-one derivatives and their absolute stereochemistry was unambiguously determined using the modified Mosher’s method [30][31][32]. One novel macrocyclic alkaloid, misenine (34), was purified from unclassified Reniera sponge collected in the Bay of Naples (Italy) [33]. Unfortunately, no report of their bioactivity has been published until now.
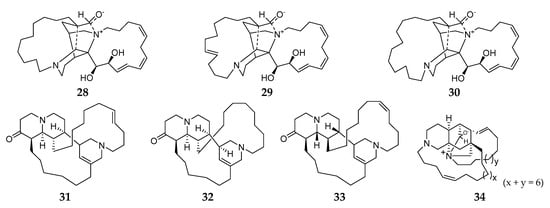
Figure 3. Macrocyclic diamines derivatives 28–34 from the subgenus Reniera.
2.1.4. Other Alkaloids
A search for antimicrobial substance(s) from an unidentified Reniera sponge from Isla Grande (Mexico) led to the isolation of one new isoindole-4,7-dione derivative (35) (Figure 4) [24], which was chemically synthesized through the cycloaddition of a nonstabilized azomethine ylide and a quinone by Parker and coworkers in 1984 [34]. Chromatography on a column of a cation exchange resin of the n-butanol soluble fraction from the acetone extracts of the sponge R. cratera afforded one simple nitrogenous compound 2-aminoimidazole (36) [35]. One cyclic depsipeptide renieramide (37) was also isolated and characterized from Reniera sp. No. 2115 collected on the Island of Santo (Vanuatu) [2]. Bioassay-guided fractionation of the MeOH extract of one marine sponge Haliclona (Reniera) sp. collected off Ulleung Island (Korea) led to the discovery of a new sphingosine (38) together with two lysophosphatidylcholines (39 and 40), which exhibited moderate cytotoxicity against a panel of five human solid tumor cell lines including A549, SK-OV-3, SK-MEL-2, XF498, and HCT15 [36].
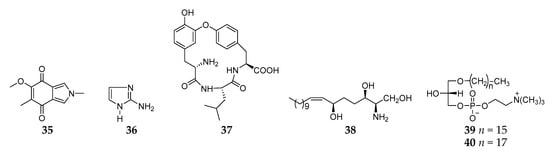
Figure 4. Other alkaloids derivatives 35–40 from the subgenus Reniera.
2.2. Terpenoids
Structurally, most of terpenoids produced by Reniera sponges are tetraterpenes except the sesquiterpenoid fulvanin 1 (41) (Figure 5) [37]. Interestingly, these tetraterpenes are carotenoid analogs, including acetylenic carotenoids (42–43), renieratene (44), isorenieratene (45), and renierapurpurin (46) from R. japonica [38][39]. It is noteworthy that two symbiotic strains, Flexibacter sps. DK30213 and DK30223, isolated from R. japonica, were found to produce zeaxanthin (47), which is one of the commonly used antioxidant agents [40]. Two new cytotoxic meroditerpenes, halioxepine B (48) and halioxepine C (49), along with halioxepine (50), were isolated from two Indonesian sponges of the genus Haliclona (Reniera) and structurally determined by QM/NMR-DFT (quantum mechanics combined with nuclear magnetic resonance parameters calculated by density functional theory approximations) analysis [41]. It is noteworthy that compound 50 had first been synthesized and the absolute configuration at position C15 was revised as S [42].
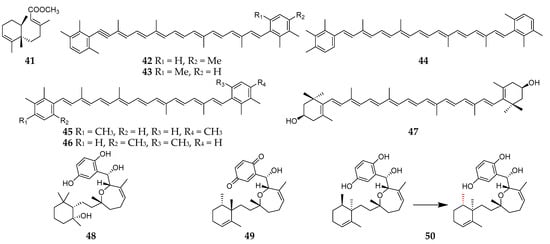
Figure 5. Terpenoids 41–50 from the subgenus Reniera.
2.3. Polyketides
2.3.1. Aromatic Polyketides
Polyketides are one of the major groups of Reniera-derived secondary metabolites, such as aromatic and aliphatic polyketides. As many as eighteen aromatic polyketides (51–67) (Figure 6) had been separated from R. fulva and R. mucosa, which were respectively collected from the Egadi Islands (Italy) and Tarifa Island (Spain) [37][43]. Compounds 52, 53, 56, and 59 possessed in vitro cytotoxicity against P388 mice lymphoma, A549 human lung carcinoma, HT29 human colon carcinoma, and MEL28 human melanoma cell lines with the same ED50 values of 5 mg/mL [43]. Moreover, compound 59 exhibited a moderate inhibitory effect on DHFR (dihydrofolate reductase) with an ED50 value of 3 mg/mL. At the concentrations from 10−4 to 10−8 M, compounds 61 and 63 were shown to be cytotoxic to the NCI-H522 nonsmall lung cancer cell line and CCRF-CEM leukemia cell line, while 54 had more selective cytotoxicity against the latter [37].
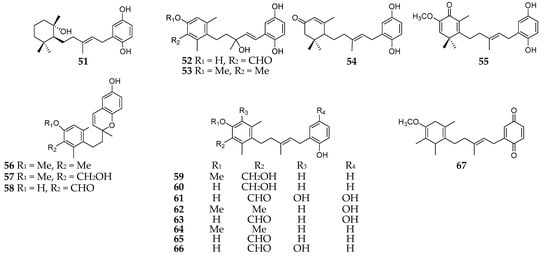
Figure 6. Aromatic polyketides derivatives 51–67 from the subgenus Reniera.
2.3.2. Aliphatic Polyketides
Linear alkynols and alkynones are the most common aliphatic polyketides detected in the marine sponge R. fulva. Fulvinol (68, Figure 7), a new long-chain diacetylenic compound, was purified from R. fulva collected at Algeciras Bay (Spain) and found to possess an inhibitory effect on P-388 mouse lymphoma, A-549 human lung carcinoma, HT-29 human colon carcinoma, and MEL-28 human melanoma cell lines with the same ED50 values of 1 μg/mL [44]. One search for secondary metabolites of R. fulva from the Mediterranean Sea resulted in the isolation of five new acetylenic compounds including debrorenierin-1 (69), renierin-1 (70), lb-dihydrorenierin-1 (71), renierin-2 (72), and 18-hydroxyrenierin-2 (73) [45].
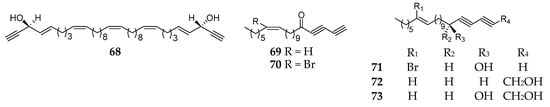
Figure 7. Aliphatic polyketides 68–73 from the subgenus Reniera.
2.3.3. Other Polyketide
One new bicyclic eicosanoid named mucosin (74) (Figure 8) was detected in the acetone extracts of R. mucosa samples, which had been collected in different areas including Blanes (Spain), Grotte de Jarr (France), Massalubrense, and Procida (Italy) [46]. Interestingly, this metabolite contains an unusual bicyclo [4.3.0] nonane skeleton with equilibrium of normal physiology in mammalian systems.
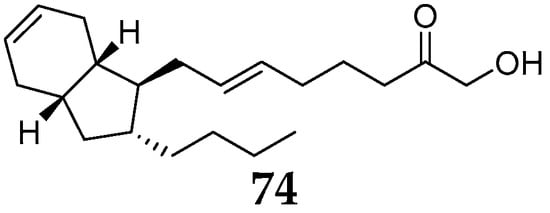
Figure 8. Other polyketide 74 from the subgenus Reniera.
2.4. Sterols
To date, a total of 27 sterol derivatives (75–101) (Figure 9) have been obtained and characterized from Reniera sponges. Chemical analysis of one unidentified sample (#063176) deposited at California Academy of Sciences Museum afforded eleven sterols (74–85) [47], and another specimen (R. sarai) collected from the Bay of Naples (Italy) resulted in the isolation of ten sterols (87–96) [48]. Using vacuum liquid chromatography (VLC), flash column chromatography, and preparative thin-layer chromatography (PTLC), β-sitosterol (97) was purified from ethyl acetate extract of Haliclona (Reneira) fascigera sponge (SPV06/12/13) collected off Samalona Island (Indonesia) [49]. Along with three alkaloids (38–40), six sterol analogs 77, 86, and 98–101 were separated from the MeOH extract of the sponge Haliclona (Reniera) sp. J01U-6 from Ulleung Island (Korea) [36].
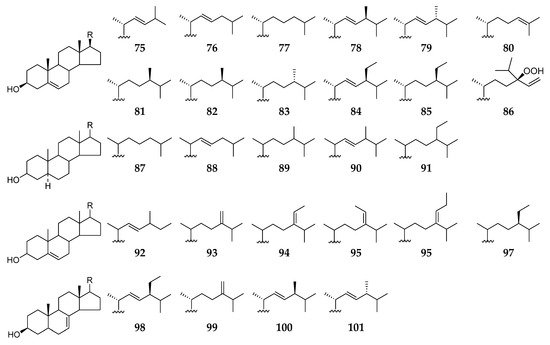
Figure 9. Sterols 75–101 from the subgenus Reniera.
2.5. Cerebrosides and Ceramide
Cerebrosides are a group of glycosphingolipids consisting of a glucose or galactose residue attached to a ceramide moiety containing one sphingoid base and an amide-linked long fatty acyl chain. These amphipathic biomolecules are important components of tissues and organs in organisms and possess a broad spectrum of biological functions such as antifungal, antitumor, antiviral, an inhibitory effect on histidine decarboxylase, and cytotoxicity [50]. Chemical analysis of the n-hexane layer of the MeOH extract of the same specimen Haliclona (Reniera) sp. J01U-6 collected off the coast of Ulleung Island (Korea) afforded twenty-one new cerebrosides (101–111, 113–122) together with one known analog (112), which possess unprecedented unsaturated or saturated long (C15–C28) alkyl chains (Figure 10) [51][52]. This was the first report on the isolation of isomeric pairs of glucocerebrosides containing saturated C15 and C19 acyl chains. Lately, the structural determination of compounds 109–112, 115, 116, 120, and 121 were well established using fast atom bombardment mass spectrometry (FAB-MS) in positive-ion mode by Hong and coworkers [53]. In addition, one ceramide (123) was separated from the same sponge SPV06/12/13 collected off Samalona Island, and its absolute structure was determined by HyperChem computational techniques [49].
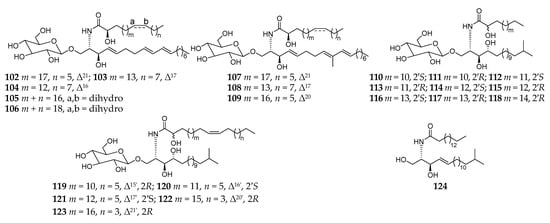
Figure 10. Cerebrosides 102–123 and ceramide 124 from the subgenus Reniera.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26041097
References
- MarinLit-A Database of the Marine Natural Products Literature. Available online: (accessed on 7 June 2020).
- Ciasullo, L.; Casapullo, A.; Cutignano, A.; Bifulco, G.; Debitus, C.; Hooper, J.; Gomez-Paloma, L.; Riccio, R. Renieramide, a cyclic tripeptide from the Vanuatu sponge Reniera n. sp. J. Nat. Prod. 2002, 65, 407–410.
- Langenbruch, P.F. Body structure of marine sponges 1. Arrangement of the flagellated chambers in the canal system of Reniera sp. Mar. Biol. 1983, 75, 319–325.
- Marine Species Identification Portal. Available online: (accessed on 24 July 2020).
- Sepcic, K.; Guella, G.; Mancini, I.; Pietra, F.; Dalla Serra, M.; Menestrina, G.; Tubbs, K.; Macek, P.; Turk, T. Characterization of anticholinesterase-active 3-alkylpyridinium polymers from the marine sponge Reniera sarai in aqueous solutions. J. Nat. Prod. 1997, 60, 991–996.
- Grandic, M.; Araoz, R.; Molgo, J.; Turk, T.; Sepcic, K.; Benoit, E.; Frangez, R. The non-competitive acetylcholinesterase inhibitor APS12-2 is a potent antagonist of skeletal muscle nicotinic acetylcholine receptors. Toxicol. Appl. Pharmacol. 2012, 265, 221–228.
- Mancini, I.; Sicurelli, A.; Guella, G.; Turk, T.; Macek, P.; Sepcic, K. Synthesis and bioactivity of linear oligomers related to polymeric alkylpyridinium metabolites from the Mediterranean sponge Reniera sarai. Org. Biomol. Chem. 2004, 2, 1368–1375.
- Grandic, M.; Zovko, A.; Frangez, R.; Turk, T.; Sepcic, K. Binding and permeabilization of lipid bilayers by natural and synthetic 3-alkylpyridinium polymers. Biorg. Med. Chem. 2012, 20, 1659–1664.
- Zovko, A.; Viktorsson, K.; Lewensohn, R.; Kolosa, K.; Filipic, M.; Xing, H.; Kem, W.R.; Paleari, L.; Turk, T. APS8, a polymeric alkylpyridinium salt blocks α7 nAChR and induces apoptosis in non-small cell lung carcinoma. Mar. Drugs 2013, 11, 2574–2594.
- Malovrh, P.; Sepcic, K.; Turk, T.; Macek, P. Characterization of hemolytic activity of 3-alkylpyridinium polymers from the marine sponge Reniera sarai. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 124C, 221–226.
- Bunc, M.; Strupi-Suput, J.; Vodovnik, A.; Suput, D. Toxic effects of head-to-tail 3-alkylpyridinium polymers isolated from the marine sponge Reniera sarai in rat. Toxicon 2002, 40, 843–849.
- Lunder, M.; Drevensek, G.; Hawlina, S.; Sepcic, K.; Ziberna, L. Cardiovascular effects induced by polymeric 3-alkylpyridinium salts from the marine sponge Reniera sarai. Toxicon 2012, 60, 1041–1048.
- Bunc, M.; Sepčić, K.; Turk, T.; Suput, D. In vivo effects of head-to-tail 3-alkylpyridinium polymers isolated from the marine sponge Raniera sarai. Pfluegers Arch Eur. J. Physiol. 2000, 440, R173–R174.
- Faimali, M.; Sepčić, K.; Turk, T.; Geraci, S. Non-toxic antifouling activity of polymeric 3-alkylpyridinium salts from the Mediterranean sponge Reniera sarai (Pulitzer-Finali). Biofouling 2003, 19, 47–56.
- Elersek, T.; Kosi, G.; Turk, T.; Pohleven, F.; Sepčić, K. Influence of polymeric 3-alkylpyridinium salts from the marine sponge Reniera sarai on the growth of algae and wood decay fungi. Biofouling 2008, 24, 137–143.
- Cuzman, O.A.; Faraloni, C.; Turchetti, T.; Camaiti, M.; Tiano, P. Influence of some natural compounds on freshwater microfoulants. Science and technology against microbial pathogens: Research, development and evaluation. In Proceedings of the International Conference on Antimicrobial Research, Valladolid, Spain, 3–5 November 2010; Mendez-Vilas, A., Ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2011; pp. 104–108.
- Chelossi, E.; Mancini, I.; Sepcic, K.; Turk, T.; Faimali, M. Comparative antibacterial activity of polymeric 3-alkylpyridinium salts isolated from the Mediterranean sponge Reniera sarai and their synthetic analogs. Biomol. Eng. 2006, 23, 317–323.
- Laville, R.; Genta-Jouve, G.; Urda, C.; Fernandez, R.; Thomas, O.P.; Reyes, F.; Amade, P. Njaoaminiums A, B, and C: Cyclic 3-alkylpyridinium salts from the marine sponge Reniera sp. Molecules 2009, 14, 4716–4724.
- Grandic, M.; Sepcic, K.; Turk, T.; Juntes, P.; Frangez, R. In vivo toxic and lethal cardiovascular effects of a synthetic polymeric 1,3-dodecylpyridinium salt in rodents. Toxicol. Appl. Pharmacol. 2011, 255, 86–93.
- Grandic, M.; Araoz, R.; Molgó, J.; Turk, T.; Sepčić, K.; Benoit, E.; Frangež, R. Toxicity of the synthetic polymeric 3-alkylpyridinium salt (APS3) is due to specific block of nicotinic acetylcholine receptors. Toxicology 2013, 303, 25–33.
- Reyes, F.; Fernandez, R.; Urda, C.; Francesch, A.; Bueno, S.; de Eguilior, C.; Cuevas, C. Njaoamines A-F, new cytotoxic polycyclic alkaloids from the haplosclerid sponge Reniera sp. Tetrahedron 2007, 63, 2432–2438.
- Urda, C.; Perez, M.; Rodriguez, J.; Fernandez, R.; Jimenez, C.; Cuevas, C. Njaoamine I, a cytotoxic polycyclic alkaloid from the Haplosclerida sponge Haliclona (Reniera) sp. Tetrahedron Lett. 2018, 59, 2577–2580.
- Kubo, A.; Nakahara, S.; Inaba, K.; Kitahara, Y. Synthesis of renierone, 7-methoxy-1,6-dimethyl-5,8-dihydroisoquinoline-5,8-dione and N-formyl-1,2-dihydrorenierone, antimicrobial metabolites from a marine sponge, Reniera species. Chem. Pharm. Bull. 1986, 34, 4056–4068.
- Frincke, J.M.; Faulkner, D.J. Antimicrobial metabolites of the sponge Reniera sp. J. Am. Chem. Soc. 1982, 104, 265–269.
- He, H.-Y.; Faulkner, D.J. Renieramycins E and F from the sponge Reniera sp.: Reassignment of the stereochemistry of the renieramycins. J. Org. Chem. 1989, 54, 5822–5824.
- Guo, Y.; Madaio, A.; Trivellone, E.; Scoganmiglio, G.; Cimino, G. Structural and stereochemical studies of saraines: Macrocyclic alkaloids of the sponge Reniera sarai. Tetrahedron 1996, 52, 8341–8348.
- Bernard, D. The Saraine Alkaloids. Alkaloids Chem. Biol. 2014, 73, 223–329.
- Defant, A.; Mancini, I.; Raspor, L.; Guella, G.; Turk, T.; Sepcic, K. New structural insights into saraines A, B, and C, macrocyclic alkaloids from the Mediterranean sponge Reniera (Haliclona) sarai. Eur. J. Org. Chem. 2011, 2011, 3761–3767.
- Garg, N.K.; Becker, M.H.; Chua, P.; Downham, R.; Douglas, C.J.; Hiebert, S.; Jaroch, S.; Matsuoka, R.T.; Middleton, J.A.; Ng, F.W.; et al. Total Synthesis of (−)-Sarain A. In Proceedings of the 232nd ACS National Meeting, San Francisco, CA, USA, 10–14 September 2006; American Chemical Society: Washington, DC, USA, 2006; p. ORGN-857.
- Cimino, G.; Spinella, A.; Trivellone, E. Isosarain-1. A new alkaloid from the Mediterranean sponge Reniera sarai. Tetrahedron Lett. 1989, 30, 133–136.
- Guo, Y.; Madaio, A.; Trivelllone, E.; Scognamiglio, G.; Cimino, G. Further studies of alkaloids from Reniera sarai: Structures of saraine-3 and isosaraine-3; absolute stereochemistry of saraine-1 and saraine-2. Tetrahedron 1996, 52, 14961–14974.
- Cimino, G.; Fontana, A.; Madaio, A.; Scognamiglio, G.; Trivellone, E. Application of two-dimensional shift correlated NMR techniques to the structure determination of an unusual marine alkaloid, isosaraine-2. Magn. Reson. Chem. 1991, 29, 327–332.
- Guo, Y.; Trivellone, E.; Scognamiglio, G.; Cimino, G. Misenine, a novel macrocyclic alkaloid with an unusual skeleton from the Mediterranean sponge Reniera sp. Tetrahedron 1998, 54, 541–550.
- Parker, K.A.; Cohen, I.D.; Padwa, A.; Dent, W. Cycloadditions of non-stabilized azomethine ylides and quinones. Synthesis of the Reniera isoindole. Tetrahedron Lett. 1984, 25, 4917–4920.
- Cimino, G.; De Stefano, S.; Minale, L. Occurrence of hydroxyhydroquinone and 2-aminoimidazole in sponges. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1974, 47, 895–897.
- Mansoor, T.A.; Park, T.; Luo, X.; Hong, J.; Lee, C.O.; Jung, J.H. A new sphingosine from a marine sponge Haliclona (Reniera) sp. Nat. Prod. Sci. 2007, 13, 247–250.
- Casapullo, A.; Minale, L.; Zollo, F. Paniceins and related sesquiterpenoids from the Mediterranean sponge Reniera fulva. J. Nat. Prod. 1993, 56, 527–533.
- Hamasaki, T.; Okukado, N.; Yamaguchi, M. Two natural acetylenic aromatic carotenoids. Bull. Chem. Soc. Jpn. 1973, 46, 1884–1885.
- Yamaguchi, M. Total syntheses of renieratene and renierapurpurin. Bull. Chem. Soc. Jpn. 1960, 33, 1560–1562.
- Miki, W.; Otaki, N.; Yokoyama, A.; Kusumi, T. Possible origin of zeaxanthin in the marine sponge, Reniera japonica. Experientia 1996, 52, 93–96.
- Tarazona, G.; Benedit, G.; Fernandez, R.; Perez, M.; Rodríguez, J.; Jimenez, C.; Cuevas, C. Can stereoclusters separated by two methylene groups be related by DFT Studies? The case of the cytotoxic meroditerpenes halioxepines. J. Nat. Prod. 2018, 81, 343–348.
- Poock, C.; Kalesse, M. Total synthesis and structure revision of halioxepine. Chem. Eur. J. 2021, 27, 1615–1619.
- Zubia, E.; Ortega, M.J.; Carballo, J.L.; Salva, J. Sesquiterpene hydroquinones from the sponge Reniera mucosa. Tetrahedron 1994, 50, 8153–8160.
- Ortega, M.J.; Zubia, E.; Carballo, J.L.; Salva, J. Fulvinol, a new long-chain diacetylenic metabolite from the sponge Reniera fulva. J. Nat. Prod. 1996, 59, 1069–1071.
- Cimino, G.; De Stefano, S. New acetylenic compounds from the sponge Reniera fulva. Tetrahedron Lett. 1977, 18, 1325–1328.
- Casapullo, A.; Scognamiglio, G.; Cimino, G. Mucosin: A new bicyclic eicosanoid from the Mediterranean sponge Reniera mucosa. Tetrahedron Lett. 1997, 38, 3643–3646.
- Lawson, M.P.; Stoilov, I.L.; Thompson, J.E.; Djerassi, C. Cell membrane localization of sterols with conventional and unusual side chains in two marine demosponges. Lipids 1988, 23, 750–754.
- Dini, A.; Piccialli, V.; Pronzato, R.; Sica, D. Sterol composition of marine sponges Stryphnus mucronatus and Reniera sarai. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1985, 81B, 111–114.
- Sapar, A.; Ahmad, A.; Soekamto, N.H.; Noor, A. Isolation and characterization of a ceramide and b-sitosterol compounds on Haliclona (Reniera) fascigera from Spermonde Archipelago. Int. J. ChemTech Res. 2017, 10, 52–61.
- Tan, R.X.; Chen, J.H. The cerebrosides. Nat. Prod. Rep. 2003, 20, 509–534.
- Mansoor, T.A.; Shinde, P.B.; Luo, X.; Hong, J.; Lee, C.O.; Sim, C.J.; Son, B.W.; Jung, J.H. Renierosides, cerebrosides from a marine sponge Haliclona (Reniera) sp. J. Nat. Prod. 2007, 70, 1481–1486.
- Park, T.; Mansoor, T.A.; Shinde, P.B.; Bao, B.; Hong, J.; Jung, J.H. New cerebrosides from a marine sponge Haliclona (Reniera) sp. Chem. Pharm. Bull. 2009, 57, 106–111.
- Ahn, Y.M.; Lee, W.W.; Jung, J.H.; Lee, S.G.; Hong, J. Structural determination of glucosylceramides isolated from marine sponge by fast atom bombardment collision-induced dissociation linked scan at constant B/E. J. Mass. Spectrum. 2009, 44, 1698–1708.
This entry is offline, you can click here to edit this entry!
