Переносчики ABC представляют собой большое семейство мембранных белков, которые транспортируют химически разнообразные субстраты через липидный бислой плазматических мембран клеток, сопровождаясь гидролизом АТФ. В настоящее время у человека известно 49 различных генов, кодирующих переносчики ABC, которые, исходя из структурных особенностей, делятся на семь подсемейств, обозначенных ABCA – ABCG.
- chronic obstructive pulmonary disease
- COPD
- atherosclerosis
- inflammation
- ABC transporters
- lipid metabolism
1. Введение
Хроническая обструктивная болезнь легких (ХОБЛ) является важной медицинской проблемой, что связано с высокой распространенностью заболевания, его влиянием на качество жизни, а также высокой частотой инвалидности и смертности [ 1 , 2 , 3 ]. Важной характеристикой ХОБЛ является разнообразие ее клинических проявлений, в основе которых лежит множество не до конца изученных патофизиологических механизмов [ 4 , 5 , 6 ]. Эта клиническая неоднородность имеет различные легочные и внелегочные характеристики, такие как развитие эмфиземы и других сопутствующих заболеваний [ 7 , 8]. Считается, что атеросклероз и ХОБЛ имеют ряд общих механизмов развития, что позволяет считать их коморбидными заболеваниями [ 11 , 12 ].
Тесная связь между ХОБЛ и атеросклерозом хорошо известна клиницистам [ 13 ]. Также хорошо известно влияние увеличения бронхиальной обструкции и увеличения числа обострений ХОБЛ на прогрессирование атеросклероза [ 15 , 16 , 17 ]. В свою очередь, представляет собой большой клинический и исследовательский интерес. Известно, что липиды плазмы крови связаны с функцией легких [ 23 , 24 , 25 ].
Интересно, что атеросклероз не часто одинаково представлен в разных группах пациентов с ХОБЛ, но более характерен для фенотипа бронхита, что предполагает наличие общих нарушенных механизмов [ 27 ]. На сегодняшний день нет четкого понимания всех процессов, связывающих развитие и прогрессирование ХОБЛ и атеросклероза, но считается, что связующим звеном является системное воспаление [ 27 , 28 ]. Многие клетки участвуют в поддержании системного воспаления, включая макрофаги, которые известны разнообразием своих функций [ 28 ]. И при ХОБЛ, и при атеросклерозе макрофаги чрезмерно инфильтрируют бронхиальную или сосудистую стенку соответственно [ 29 ,30 , 31 ].
They are also key participants in the initiation and progression of atherosclerosis, which is determined by their role in the uptake of modified lipoproteins in the arterial walls, and the production of inflammatory mediators and matrix metalloproteinases that contribute to the instability of atherosclerotic plaques [37]. A violation of the normal homeostasis of cholesterol in macrophages and a massive accumulation of its esters in lipid droplets lead to the acquisition of the so-called “foam cell” phenotype by cells [38]. Removing excess cholesterol from cells is called reverse cholesterol transport. Currently, the role of lipid metabolism disorder in the development of atherosclerosis is sufficiently understood and supported by the results of numerous studies.
Recent data have shown that lipid metabolism disorders are involved in many cross-immunometabolic pathways and participate in the development of COPD [39,40]. For example, the association of low body weight in COPD with high mortality is known, which has been called the “obesity paradox” [42,43,44]. Despite the fact that the prognostically significant cause of weight loss in this category of patients is the loss of muscle mass rather than fat, the importance of lipid metabolism disorders in COPD patients is not in doubt. As it is well known, excess body weight correlates well with an unfavorable prognosis in cardiovascular diseases of an atherosclerotic nature, but it has an inverse relationship with the prognosis of COPD [45], which is an important difference that characterizes the features of lipid metabolism in these patients.
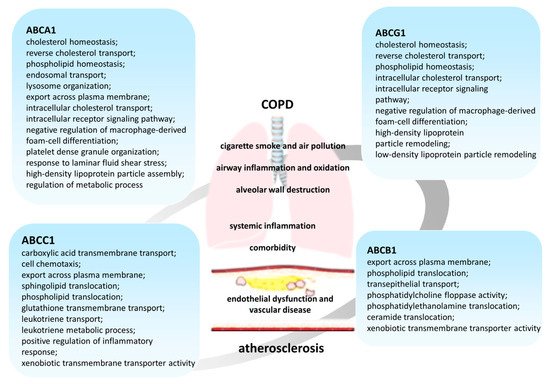
The results of studies published in recent years suggest that cellular lipid metabolism has numerous cross-links with the immune response [46]. In this regard, disorders of lipid metabolism mediated by ATP-binding cassette (ABC) transporters are of clinical interest (Figure 1).
ABC transporters are a large family of membrane proteins that transport chemically diverse substrates through the lipid bilayer of cell plasma membranes while accompanied by ATP hydrolysis [47,48]. Currently, 49 different genes encoding ABC transporters are known in humans, which, based on the structural features, are divided into seven subfamilies, designated ABCA–ABCG [49,50,51,52,53].
Moreover, lipid carriers are present in all subfamilies of ABC transporters, which emphasizes the importance of lipid transport [54,55,56]. The need for special transport mechanisms for lipids is due to their insolubility in water [54]. In addition, cell membranes are heterogeneous in their lipid composition [55]. Unlike flippases, ABC transporters are responsible for the movement of lipid substrates from the inner leaflet of the plasma membrane to the outer leaflet, where lipids must be desorbed or diffuse to extracellular lipid acceptors [54].
The accumulated data in recent years indicate an important role of cholesterol in the functioning of ABC transporters. Cholesterol is a key molecule of the plasma membrane that provides stabilization of the spatial structure of the lipid bilayer [57,58]. Such a spatial arrangement of the molecules is considered to contribute to the participation of cholesterol in the regulation of the function of transmembrane proteins through two main mechanisms: direct interaction of the sterol with specific protein binding sites and indirect influence on the biophysical properties of the membrane [57,58,59,60]. This information is of great clinical and research interest since it allows us to assess the significance of separate processes in the development and progression of comorbid COPD and atherosclerosis in a different way.
The objective of this review was the analysis of the role of lipid metabolism disorders in the pathogenesis of the comorbid course of COPD and atherosclerosis and the participation of representatives of ABC transporters in these processes.
2. Subfamily of ABCB Transporters
Members of the ABCB subfamily are well known to clinicians and researchers for their role in the development of multiple drug resistance. At the same time, a number of members of the ABCB subfamily are also characterized by their ability to transport lipids, such as ABCB1 [55,56,147,148], or ABCB4, which participates in the translocation of phosphatidylcholine lipids into bile [56,149,150].
(P-glycoprotein, MDR1 (multidrug resistance 1 gene)), cloned by J.R. Riordan in 1985, was originally described as a major participant in the mechanism of multiple drug resistance to chemotherapeutic agents of colchicine-resistant Chinese hamster ovarian cells [151,152,153]. To date, ABCB1 is one of the most well-studied ABC transporters (Figure 2) [55,56,154,155]. Its broad substrate specificity allows it to carry out the transport of chemically diverse molecules, including medicinal substances [56,147,156]. However, the mechanism by which ABCB1 can recognize different chemical structure substrates remains largely unknown.
It is known that ABCB1 participates in lipid transport [55,56,157], moving lipids from the inner to the outer leaflet of the plasma membrane of cells [56,76]. It was shown that cholesterol is recognized and transported as an endogenous ABCB1 substrate [158,159,160]. Moreover, ABCB1 not only transports cholesterol across the membrane but also its functional activity is modulated by cholesterol in the membrane [159,161]. A direct interaction between lipid molecules and ABCB1 was revealed [56,76,162].
It was shown that the predominant localization of ABCB1 in lipid rafts is necessary for its functioning [160,163,164]. Cholesterol depletion, which leads to the destruction of lipid rafts, disrupts the membrane localization of ABCB1 and reduces its transport activity [165,166], which leads to the intracellular accumulation of drugs in cells [159]. There is a supposition that sterols may interact with ABCB1 and modulate its structure and function by occupying part of the drug-binding pocket or by binding to assumed consensus cholesterol-binding motifs (CRAC/CARC) that are located in the transmembrane domains [55,58,60,167,168]. Thus, the effect of cholesterol on the transport activity of ABCB1 is of great clinical interest.
Although the lungs are not among the organs with a high expression of ABCB1, the protein is found in the bronchi, where it is mainly localized on the apical surface of the ciliated epithelial cells, the apical and lateral surfaces of the serous cells of the bronchial glands, and the lumen surface of the endothelial cells of the bronchial capillaries, but it is not found in the mucus-secreting goblet cells [169,170]. In the alveoli, ABCB1 is expressed in type I alveolar epithelial cells [171,172].
ABCB1 is activated during differentiation from monocytes to macrophages and is sensitive to activation by LXR (liver X receptor) agonists [173,174]. In addition, it is also present in macrophages, with higher expression in the M2 subtype compared to M1 macrophages [163,175,176,177]. It has been reported that tobacco smoke components affect the expression and functional activity of ABCB1 [172,178]. A decrease in the expression ofABCB1in the cells of smokers compared to non-smokers was shown.
It should be noted that the inhaled administration of glucocorticoids in patients with severe COPD may cause existing differences in the expression ofABCB1in the lungs [179].
It has been shown that polymorphism of theABCB1gene can affect the effectiveness of COPD therapy [180] and mediate extrapulmonary complications of the disease [181]. In addition, elevated levels of mRNA ABCB1 were found in the tissues in atherosclerosis, which allows us to suggest a role of ABCB1 in the development of atherosclerotic lesions in vivo [174,182].
The expression of mRNA ABCB4 in monocytes and macrophages is also shown [184,185], and the ABCB4 of macrophages prevents the formation of “foam cells,” reducing the accumulation of lipids and providing an atheroprotective function [185]. A lower serum HDL cholesterol level was observed in Abcb4 knockout mice fed with normal food, which confirmed the effect of ABCB4 on cholesterol metabolism [185,186]. Since ABCB4 is a carrier of phosphatidylcholine from the inner to the outer sheet of the plasma membrane, violations of the functional activity of the protein can probably lead to asymmetry of the phospholipid membrane of macrophages [185,187]. Thus, ABCB4 can indirectly contribute to atherogenesis by affecting the accumulation of lipids in macrophages as a result of modulation of the lipid membrane asymmetry [185].
The results of bioinformatics analysis indicate that exposure to cigarette smoke is associated with increased levels ofABCB6expression in bronchial epithelial cells, while smoking cessation is associated with lowerABCB6expression [134].
Literature data suggest that ABCB6 protects cells from oxidative stress by modulating cytosolic reactive oxygen species [188,189]. Despite this, an increase in the expression ofABCB6may also have negative effects on the form of increased resistance to chemotherapeutic agents [134].
It is known that platelets participate in the pathogenesis of atherosclerosis [192,193]. ligand 4), on the surface of endothelial cells, facilitating the recruitment of leukocytes to inflammatory foci, and also form aggregates with neutrophils and monocytes, which plays a key role in the production of inflammatory cytokines, the biosynthesis of leukotrienes, and the production of reactive oxygen species [193,194,195,196]. A high level of ABCB6 is observed in megakaryocyte progenitor cells and its deficiency leads to an increase in the number of circulating platelets, the interaction of platelets with inflammatory leucocytes, and the accelerated development of atherosclerosis [198].
Thus, representatives of the ABCB family are of great clinical importance, not only due to their involvement in the efflux activity for xenobiotics but also due to the movement of endogenous substrates.
3. Subfamily of ABCC Transporters
ABCC1 (MRP1 (multidrug resistance-associated protein 1)) was initially identified as a glutathione-conjugate transporter [55,199,200], but later its participation in lipid transport [55,201] and inflammatory responses was described (Figure 2).
In the lungs, ABCC1 is found in alveolar macrophages, as well as in bronchial epithelial cells [202], and its expression levels differ in different parts of the respiratory tract [134]. Localization of the protein in the cells of the distal parts of bronchi was already associated with participation in the development of COPD [203]. Interestingly, in contrast to ABCB1, ABCC1 in basal cells was distributed along the entire circumference of the plasma membrane, and in ciliated cells, it is localized on the basolateral surface [204], which predetermines the functional differences of the transporters [205].
ABCC1 is localized in cholesterol-rich actin-dependent lipid rafts, which determines the functional dependence of the efflux activity of the transporter on cortical actin However, there is little information in the literature about the participation of cholesterol in the modulation of the ABCC1 transport function. In one study, it was found that the functional activity of the transporter decreased with a decrease in the level of cellular cholesterol [208], but in other studies, it was shown that cholesterol was not a necessary factor for the function of ABCC1 and, apparently, did not participate in the mechanisms of functional connection of ABCC1 with lipid rafts [209].
It was shown that ABCC1 can protect the lungs from developing COPD by reducing the oxidative stress caused by smoking, preventing the accumulation of toxic metabolites [210,211]. It is assumed that the expression of theABCC1gene is regulated by a feedback mechanism since it is associated with oxidative stress and exposure to toxins caused by exposure to cigarette smoke and contributes to the enhancement of antioxidant activity in the epithelial cells of the respiratory tract [134].
A lowerABCC1expression in the bronchial epithelium of COPD patients compared to healthy former smokers was shown [218]. Expression was also lower in patients with severe COPD than in those with mild or moderate COPD. In contrast, data from a recent bioinformatics analysis show an increase in the expression of theABCC1gene in smoking patients with COPD compared to people who do not have COPD [134]. The available contradictory information on the expression of the transporter in patients with COPD may be associated with the use of glucocorticoids in the severe course of disease [214].
Leukotrienes, which are a group of highly effective lipid mediators, are important participants in the antibacterial protection of the lungs. Leukotriene LTC4occupies an important place among the physiological substrates of ABCC1 [200,223,224]. In this connection, the transport activity of ABCC1 may participate in pulmonary inflammation. They showed reduced pneumococcal growth in the lungs and strongly reduced mortality, which was associated with an increase in circulating LTB4, which is a powerful chemoattractant for neutrophils and increases the activity of phagocytic cells [226,227,228].
In addition to these data, it was shown that mice with triple knockout of theMrp1andMdr1a/1bgenes were more susceptible to the development of COPD. These mice had lower levels of IL-8 production and showed an almost complete absence of inflammatory cells in response to cigarette smoke [215,223]. The impaired inflammatory response was likely associated with lower LTC4excretion [231].
Data about the participation of ABCC1 in the export of sphingosine-1-phosphate (S1P), which is a lipid mediator that is involved in many processes, including inflammation, angiogenesis, apoptosis, and macrophage function, are of great importance [232,233,234,235,236,237,238,239,240,241]. S1P also regulates the integrity of the endothelial barrier by modulating the endothelial cytoskeleton [242].
It was shown that S1P levels were elevated in the induced sputum of COPD patients compared to non-smokers [243]. It was suggested that S1P may be a participant in defective phagocytosis by macrophages in COPD [243,244].
Interestingly, the impairment of S1P metabolism is an important factor determining the emphysematous phenotype in COPD [245].
In addition to ABCC1, ABCA1 and ABCG2 also participate in S1P transport [234,246,247].
It has been determined that ABCC1 plays a definite role in the regulation of vascular endothelial homeostasis and arterial blood pressure by inducing the release of glutathione from vascular endothelial cells [248,249,250]. ABCC1 is found in large quantities in vascular smooth muscle cells, which make up the majority of vascular wall cells and are involved in the process of atherosclerosis. ABCC1 acts as a transporter for substances such as glutathione, oxidized glutathione, and leukotriene C4(LTC4) [248,250,251], which are potentially essential for regulating the production of reactive oxygen species in vascular cells. In addition, modulation of ABCC1 expression in human aortic endothelial cells affects vascular function [248,251].
Thus, ABCC1 demonstrates involvement in the development of COPD and atherosclerosis. However, the mechanisms of this participation require further study.
4. Subfamily of ABCG Transporters
It is known that many proteins of the ABCG subfamily also participate in lipid homeostasis [252]. An important role in lipid metabolism is assigned to ABCG1 and ABCG4 transporters, which are half-type ABC proteins since they consist of only one transmembrane domain and one nucleotide-binding domain. For activation to occur, the protein must form a dimer (homodimer or heterodimer) or even an oligomer depending on the function [253,254,255,256,257].
It is expressed in many cell types, including myeloid cells, lymphocytes, epithelial, and endothelial cells of various organs [258,259,260,261]. It is known that, like ABCA1, ABCG1 removes excess cholesterol from peripheral cells, saturating HDL with it and protecting cells from sterol overload [257,263,264]. In the lungs,ABCG1is expressed in various cell types, including alveolar macrophages, epithelial cells, and type II pneumocytes [258,270,271]. The absence of ABCG1 leads to progressive chronic lung inflammation, which is associated with impaired regulation of intracellular cholesterol levels [121,271,272].
It was shown that Abcg1−/−macrophages were characterized by increased production of proinflammatory cytokines IL-6, IL-1β, IL-1α, and IL-12, and a decrease in anti-inflammatory cytokine IL-10 [272,274], which is associated with the lipid load of macrophages. Elevated levels of matrix metalloproteinases MMP-8 and MMP-12 were also found out in the lungs ofAbcg1−/−mice [272], they destroy the extracellular matrix, are overexpressed in COPD patients, and are associated with airway inflammation and remodeling [275,276].
The information about the participation of ABCG1 in the polarization of macrophages is important. Experimental data indicate that ABCG1 deficiency contributes to the proinflammatory M1 polarization of human macrophages, and the molecular mechanism is probably mediated via the Akt signaling pathway [277]. Moreover, this phenotypic shift is more pronounced when the diet of mice was similar to the Western one [269]. According to the existing concepts, most of the cells in the center of the atherosclerotic plaque are M1 macrophages.
It is believed that ABCG1 participates in the apoptosis of cells, including macrophages [97,257,278,279]. It was determined that macrophages of miceAbcg1−/−have an increased ability to absorb apoptotic cells, accumulate lipids, and become apoptotic [274,280,281].
It is known that apoptosis plays an important role not only in the development of COPD but also in the pathogenesis of atherosclerosis: it is antiatherogenic in the early foci of lesions, and the apoptosis of macrophages contributes to atherogenesis in progressive lesions [97]. The mechanism of ABCG1 participation in apoptosis is probably determined by its cholesterol transport activity and its functioning as an inducer or inhibitor of apoptosis depends on the localization of the transporter on the plasma membrane or intracellular membranes of organelles [97].
Taking into account the presence of the ABCG1-transporter in macrophages (“foam cells”) in human atherosclerotic plaque, it was assumed that macrophagic ABCG1 plays an important role in the development of atherosclerotic lesions This assumption was confirmed in numerous studies [174,184,277,282,283,284]. The participation of ABCG1 in lipid homeostasis was analyzed by a number of authors who demonstrated that mRNA expression and ABCG1 synthesis in macrophages can be induced by cholesterol loading [97,184,284,285]. Thus, ABCG1 is also important for intracellular cholesterol transport [286,287].
Another member of the subfamily, ABCG4 has 69% identity and 84% similarity in amino acid composition to ABCG1 and mediates the outflow of cholesterol to However, unlike ABCG1, the expression of ABCG4 is limited. The transporter is found out in the brain and hematopoietic organs [260,288,289,290,291]. Another important difference is that the activation of LXR induces the expression of Abcg1, but does not affect the expression of Abcg4 [290].
The obtained results allowed for suggesting the role of ABCG1 and ABCG4 transporters in cell proliferation, apoptosis, and immune responses, and that these different processes may be related to the regulation of lipid metabolism [262,292,293].
Although ABCG4 is not expressed in macrophage foam cells, its potential role in atherogenesis is described [294]. It is known that ABCG4 inhibits the proliferation of megakaryocyte progenitor cells via reducing the transmission of thrombopoietin receptor signals in lipid rafts [257,295]. In addition to increased atherogenesis, arterial thrombosis was found in mice withAbcg4gene knockout, which correlated with an increase in the number of reticular platelets, platelet complexes, leucocytes, and microparticles of platelet origin, which have proven pro-atherosclerotic and prothrombotic properties. Researchers associated increased platelet production caused by impaired cholesterol metabolism in progenitor cells with accelerated atherogenesis and arterial thrombosis [67,295].
ABCG2 (BCRP, breast cancer resistance protein) is another representative of the ABCG subfamily and is most commonly associated with drug excretion [55,296]. BCRP has been found in many organs and tissues, including the endothelium of venous vessels and capillaries, where it performs a protective and barrier function [297].
The expression of ABCG2 in the lungs is low and decreases in the trachea– large bronchi–small bronchi series, but the protein is found in the epithelial layer and seromucinous glands, as well as in the endothelial cells of capillaries [298]. ABCG2 is also expressed in alveolar pneumocytes and is mainly localized on the apical membrane and, to a lesser extent, in the cytosol and in the cell nuclei, where, according to some assumptions, it acts as a transcription factor, regulating gene expression. During differentiation from alveolar pneumocytes of type II to alveolar pneumocytes of type-I-like phenotype, the expression of
The role of BCRP/ABCG2 in the lungs is completely unknown. Furthermore, lung pathologies in Abcg2−/−mice have not been reported yet [176,302,303,304].
It has been shown that ABCG2 in lung tissues is responsible for the formation of the SP-phenotype (side population) of lung cancer cells, which have high efflux activity [305].
It was found in numerous studies that BCRP also participates in the transport of sterols, and cholesterol can stimulate the ATPase activity of the transporter [55,294,306,307]. This was confirmed by the fact that a change in the structure of lipid rafts directly leads to the redistribution of the BCRP protein in areas with a higher cholesterol density. With a decrease in the cholesterol content in the lipid rafts, the content of BCRP substrates in the cells increases, which indicates the inhibition of the transporter protein. The restoration of cholesterol and its saturation of lipid rafts leads to the normalization of the functional activity of the transporter [308,309].
ABCG5 and ABCG8 play a role in lipid metabolism and mediate the outflow of cholesterol and sitosterol from the intestinal walls and hepatocytes to the bile duct and intestinal lumen [159,310]. There is no information in the literature about the participation of these transporters in lung function. However, it is known that mutations of theABCG5andABCG8genes cause sitosterolemia, which is characterized by an increase in the absorption of plant and fish sterols, and their reduced biliary excretion leads to an increase in the level of toxic sitosterols in the blood and the early development of atherosclerosis and myocardial infarction. The participation of ABCG5 and ABCG8 in atherogenesis may also consist in the fact that they provide trans-intestinal excretion of cholesterol, i.e., an alternative non-biliary route of its excretion [311].
Таким образом, представители подсемейства участвуют в транспорте липидов и множества важных функций. В патогенезе ХОБЛ и атеросклероза ABCG1 является одним из наиболее интересных, поскольку нарушения его транспортной активности нарушают ряд процессов, связанных с возбуждением.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22136711
