Neuroendocrine neoplasms (NEN) are most often located in the lung and in the digestive tract. They are defined by the expression of specific biomarkers, such as synaptophysin and chromogranin A (CGA), which can be absent in high-grade NEN. Grade 3 Neuroendocrine Tumors are a subgroup of NEN presenting with well-differentiated morphology and high proliferation rate (Ki-67>20%).
- neuroendocrine neoplasms
- well-differentiated grade-3
1. Introduction
2. Specifics
NET G−3 are rare tumors showing specific features of clinical interest. Their prognosis seems closer to that of NET G−2 rather than that of NEC, but with a worse OS. If in doubt, pathologic reassessment by a NEN expert should be easily proposed, especially for pancreatic lesions where NET G−3 more often arise. Due to their rarity and the specific management they require, NET G-3 treatment should always be discussed in NEN expert meetings. Functional imaging plays a crucial role in both diagnosis and treatment management of NEN, and dual tracer imaging should easily be proposed for NET G-3 patients. To this date, available treatments include mainly surgery and chemotherapy with alkylant-based regimen and sometimes platinum-based regimen (most of the data coming from pancreatic series). There are promising results with targeted therapies and PRRT which need confirmation in larger populations. Trials with ICI are still ongoing, and the combination of chemotherapy and ICI should be explored in the future for more antitumoral activity. Based on the available literature and on the 2020 European recommendations, we propose the following algorithm for the therapeutic management of digestive NET G-3 [127] (Figure 1).
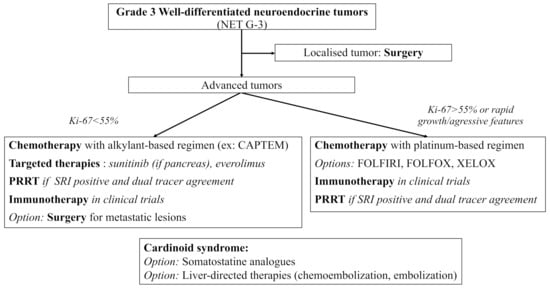
Currently, molecular biology and theranostics seem the two most promising fields of research to help individualize and standardize treatment for NET G−3. Whole genomic sequencing of PanNET G−3 samples could help identify new structural rearrangements or mutations. Data in molecular biology is also urgently needed in non-pancreatic NET G−3. Other diagnostic tools, such as the NET test, a multianalyte liquid biopsy measuring NET gene expression, might also help individualize NET G−3 in the future. Finally, dual tracer imaging should always be discussed in patients with NET G-3 to help select patients for PRRT.
Overall, it is important to highlight that our entry has limitations due to the scarcity of available studies for this newly introduced entity. Additionally, the population samples are very small. Therefore, the main conclusions on NET G-3 characteristics and specific treatment will need to be confirmed in future larger trials. In order to deal with recruitment difficulty due to the rarity of these tumors, international collaborations should be encouraged.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13102448
