Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Virology
Tilapia Lake Virus Disease (TiLVD) is a new disease caused by Tilapia tilapinevirus, or tilapia lake virus (TiLV), which is a linear, negative-sense single-strand RNA virus containing ten segments; it has a total genome size of 10,323 kb.
- Tilapia tilapinevirus
- TiLV
- Mozambique tilapia
- infection
- pathology
1. Introduction
Tilapia Lake Virus Disease (TiLVD) is a new disease caused by Tilapia tilapinevirus, or tilapia lake virus (TiLV), which is a linear, negative-sense single-strand RNA virus containing ten segments; it has a total genome size of 10,323 kb [1][2]. Since 2014, TiLV has been reported on four continents, North America, South America, Asia, and Africa [2][3][4]. All stages of tilapia, from fry to adult and broodstock, are susceptible to TiLV, with mortality ranging from 5% to 100% [2][4][5][6][7][8][9]. TiLVD has been most extensively reported and studied in several species of tilapia, including Nile tilapia (Oreochromis niloticus) [5][8][10][11][12][13][14], hybrid tilapia (Oreochromis niloticus × O. aureus) [4], gray tilapia (O. niloticus × O. aureus) [15], and red hybrid tilapia (Oreochromis sp.) [8][16]. Some other species of fish are also susceptible to TiLV, including giant gourami (Osphronemus goramy), which showed a high mortality rate during laboratory challenge [17]; the virus was also detected in wild river barb (Barbonymus schwanenfeldi) in Malaysia [18]. TiLV primarily affects tilapia and its hybrid species, while most other freshwater fish are resilient to TiLV infection, including snakeskin gourami (Trichogaster pectoralis), iridescent shark (Pangasianodon hypophtthalmus), walking catfish (Clarias macrocephalus), striped snake-head fish (Channa striata), climbing perch (Anabas testudineus), common carp (Cyprinus carpio), silver barb (Barbodes gonionotus), Asian sea bass (Lates calcarifer) [17], and Indian major carp (Labeo rohita) [19].
Tilapias of the genus Oreochromis are a popular species for aquaculture in several regions of the world; three of the most common species are Nile tilapia (Oreochromis niloticus), blue tilapia (Oreochromis aureus), and Mozambique tilapia (Oreochromis mossambicus) [20][21][22][23][24]. Mozambique tilapia is known to grow more slowly, mature at a young age, and be more tolerant to wide salinity fluctuations than Nile tilapia [21][22][23][24][25]. Therefore, Mozambique tilapia has been used in breeding to improve the saltwater and cold tolerance of other tilapias [21][26][27]. The species has also been used as a model to study environmental factors [28][29] and response to pathogens [30]. Mozambique tilapia is native to the river of South Africa [31], but its numbers have decreased due to introduction of invasive fish species, including Nile tilapia. This invasion has resulted in the extirpation of Mozambique tilapia due to habitat competition and hybridization [32][33][34][35][36][37]. The Mozambique tilapia is now therefore considered a globally vulnerable species and is on the International Union for Conservation in Nature (IUCN) Red List [38]. In addition to habitat competition, pathogen carrying and spill over between native and invasive fish species will result in decreasing numbers of the native species. The susceptibility of Mozambique tilapia to pathogens that lead to massive decreases in number is therefore essential to investigate.
Although there is no direct experimental study on the susceptibility of Mozambique tilapia to TiLV, one previous study suggested that TiLV can replicate and infect their cells [39]. Furthermore, the natural infection of red hybrid tilapia with a Mozambique tilapia genetic background with TiLV (O. niloticus × O. mossambicus) has previously been reported [40]. Given the genetic similarities between Nile tilapia and Mozambique tilapia, there is a significant possibility that TiLV may cause infection in this species.
2. Susceptibility of Mozambique Tilapia to Tilapia Lake Virus
At 28 dpc, the cumulative mortality in Mozambique tilapia with low (1 × 103 TCID50/mL) and high (1 × 105 TCID50/mL) concentration of TiLV was 48.89% and 77.78%, respectively (Figure 1). At 4 dpc, moribund Mozambique tilapia developed clinical signs of TiLV infection, including skin hemorrhage, lethargy, pale skin, abdominal swelling, skin erosion, congestion around the eye, and exophthalmos (Figure 2). Notably, mortality in fish exposed to high TiLV concentration began at 4 dpc, while the mortality in fish exposed to low TiLV concentration did not start until 7 dpc. Mortality in both low TiLV and high TiLV-exposed fish ended at 19 dpc (Figure 1). Intraperitoneal challenges (IP) of red hybrid tilapia with TiLV at 1 × 105 TCID50/mL were conducted to compare clinical signs, pathology, and mortality patterns. Similar to the Mozambique tilapia, the red hybrid tilapia also showed skin hemorrhage, swimming at the bottom of the tank, skin and fin erosion, exophthalmos, and scale protrusion, with the first mortality at 6 dpc. At the end of the experiment, the cumulative mortality of the red hybrid tilapia was 66.67% (Figure 1). No clinical signs and mortality developed in the control Mozambique and red hybrid tilapia.
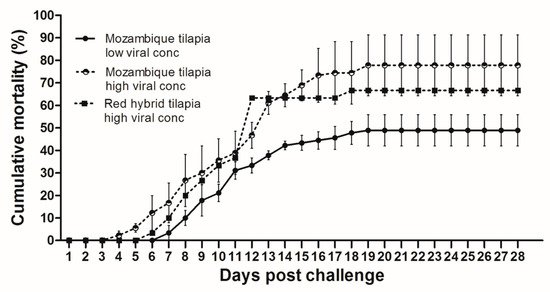
Figure 1. Cumulative mortality after Tilapia lake virus (TiLV) infection in Mozambique tilapia (Oreochromis mossambicus) and red hybrid tilapia (Oreochromis spp.). Fish were intraperitoneally challenged with TiLV at low viral concentration (1 × 103 TCID50/mL) and high viral concentration (1 × 105 TCID50/mL) per fish. Graph represents mortality in each group with triplicates, 30 fish per tank (a total of 90 fish/group). Mortality was recorded for 28 days.
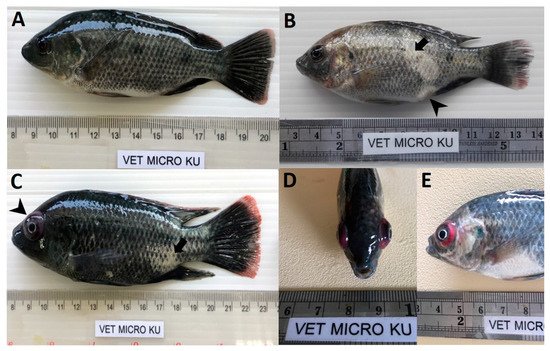
Figure 2. Clinical signs of TiLV infection in Mozambique tilapia (Oreochromis mossambicus). (A) Control uninfected fish. (B) Fish infected with TiLV at low viral concentration (1 × 103 TCID50/mL), skin erosion (arrow) and abdominal swelling (arrowhead). (C) Fish infected with TiLV at high viral concentration (1 × 105 TCID50/mL), skin erosion (arrow) and exophthalmos (arrowhead). (D,E) Fish infected with TiLV at high viral concentration (1 × 105 TCID50/mL), exophthalmos and congestion of the eye.
3. Confirmation of TiLV Infection in Moribund Mozambique Tilapia
To further confirm the susceptibility of Mozambique tilapia to TiLV, spleens were collected from low and high TiLV-challenged fish at 3, 6, and 12 dpc. As shown in Table 1, TiLV was detected in 3 out of 5 fish (60%) and 4 out of 5 fish (80%) exposed to the low concentration of TiLV at 3 and 6 dpc, respectively, with the virus concentration ranging from 9.7 × 10–4.8 × 105 copies per μg of total RNA. No TiLV was detected in the fish exposed to the low concentration at 12 dpc. Conversely, all of the fish exposed to high concentration of TiLV had TiLV in their spleen as early as 3 dpc (1.1 × 104–3.5 × 104 copies per μg of total RNA), which remained detectable in all fish until 12 dpc (3.3 × 105–2.3 × 106 copies per μg of total RNA). Throughout the studied period, no TiLV was detected in the unchallenged fish.
Table 1. Persistence of TiLV genomic RNA in spleen of challenged Mozambique tilapia.
| Positive Samples/Total Sample | |||
|---|---|---|---|
| (Number of Viral Copies/µg of Total RNA) | |||
| Day Post Challenge (DPC) | Control | Low TiLV Conc. | High TiLV Conc. |
| (1 × 103 TCID50/mL) | (1 × 105 TCID50/mL) | ||
| 3 | 0/5 | 3/5 | 5/5 |
| (9.7 × 10–1 × 103) | (1.1 × 104–3.5 × 104) | ||
| 6 | 0/5 | 4/5 | 5/5 |
| (1.3 × 103–4.8 × 105) | (2.7 × 102–2.6 × 106) | ||
| 12 | 0/5 | 0/5 | 5/5 |
| (3.3 × 105–2.3 × 106) | |||
4. Histopathological Finding and In Situ Hybridization in the Challenge Fish
Normal hepatocytes with adequate glycogen storage were found in the liver of control fish (Figure 3A,C). No extensive histopathological changes were found in the livers and spleens of Mozambique tilapia that were challenged by the low concentration of TiLV (Figure 3C,D). However, severe histopathological changes could be found in red hybrid tilapia and Mozambique tilapia exposed to high concentration of TiLV at 6 dpc. Depletion of glycogen storage, loss of sinusoid structure, presence of syncytial giant cells containing multiple nuclei, and intracytoplasmic inclusion bodies were observed in the livers of both species (Figure 3A,E). A few melanomacrophage centers (MMCs) and normal lymphocytes surrounded by a sheath of reticular cells were observed in the spleen of the control red hybrid and Mozambique tilapia (Figure 3B,D), while an abundance of MMCs and depletion of red blood cells were observed in the spleens of the fish exposed to the high TiLV concentration (Figure 3B,F). No remarkable change was observed in the gills or intestines of the challenged fish. The localization of TiLV RNA in infected tissues including liver, intestines, heart, and gills was further confirmed by ISH. Intriguingly, positive signals with green coloration were clearly observed in all tested Mozambique tilapia tissues exposed to the high TiLV concentration, while positive signals were noted to a lesser extent in liver and intestines of Mozambique tilapia exposed to the low TiLV concentration. Conversely, no positive hybridization signal was detected in either the infected tissues exposed to a non-irrelevant probe or the uninfected control tissue samples (Figure 4).
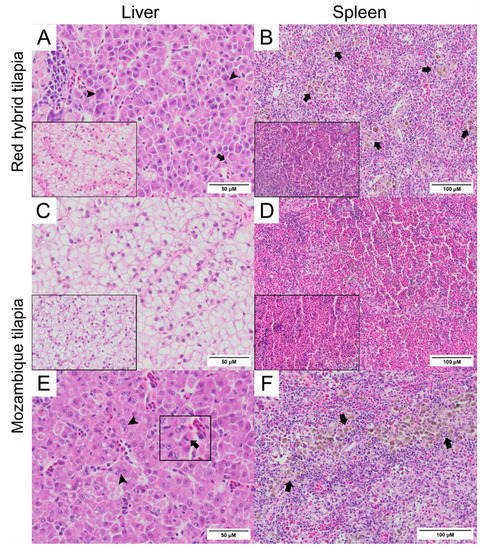
Figure 3. Histopathology of liver and spleen of red hybrid tilapia (Oreochromis spp.) and Mozambique tilapia (Oreochromis mossambicus) after 6 days post TiLV challenged. (A) Liver of red hybrid tilapia challenged with high TiLV concentration; depletion of glycogen, syncytial cells formation (arrowheads) and intracytoplasmic inclusion bodies (arrows), normal liver (inlet). (B) Spleen of red hybrid tilapia challenged with high TiLV showed depletion of red blood cells and increased melanomacrophage centers (MMCs) (arrows), normal spleen of red hybrid tilapia (inlet). (C) Liver of Mozambique tilapia challenged with low TiLV concentration, normal liver of Mozambique tilapia (inlet). (D) Spleen of Mozambique tilapia challenged with low TiLV concentration, normal spleen of Mozambique tilapia (inlet). (E) Liver of Mozambique tilapia challenged with high TiLV concentration; glycogen depletion, syncytial giant cells contained multiple nuclei (arrowheads) and intracytoplasmic inclusion bodies (inlet, arrows). (F) Spleen of Mozambique tilapia challenged with high TiLV concentration, infiltration of MMCs (arrows).
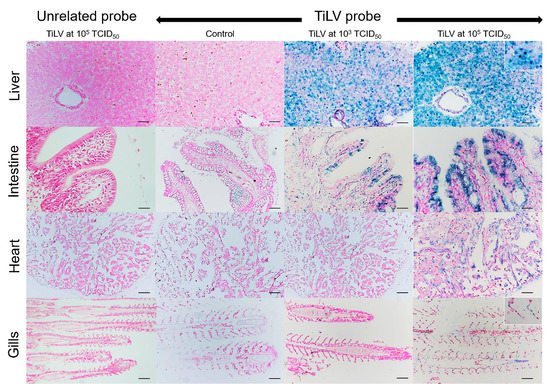
Figure 4. In situ hybridization (ISH) of TiLV RNA detection in Mozambique tilapia inoculation of TiLV at 1 × 103 TCID50/mL and 1 × 105 TCID50/mL concentrations. The TiLV RNA was detected in liver and intestinal epithelium of TiLV-infected Mozambique tilapia in both inoculation concentrations. The TiLV RNA labelling was evidence in the nucleus of hepatocytes (above inset), intestinal epithelium, myocardium, and gills epithelium (lower inset) of experimental Mozambique tilapia inoculation with TiLV at 1 × 105 TCID50/mL concentration. No evidence of hybridization signal was observed in the control fish and in the TiLV-infected Mozambique tilapia sections inoculation with CBoV-2 probe (unrelated probe). Bars indicate 120 µm.
This entry is adapted from the peer-reviewed paper 10.3390/v13061104
References
- Bacharach, E.; Mishra, N.; Briese, T.; Zody, M.C.; Kembou Tsofack, J.E.; Zamostiano, R.; Berkowitz, A.; Ng, J.; Nitido, A.; Corvelo, A.; et al. Characterization of a Novel Orthomyxo-like Virus Causing Mass Die-Offs of Tilapia. MBio 2016, 7, e00431-16.
- Surachetpong, W.; Roy, S.R.K.; Nicholson, P. Tilapia lake virus: The story so far. J. Fish Dis. 2020, 43, 1115–1132.
- Ahasan, M.S.; Keleher, W.; Giray, C.; Perry, B.; Surachetpong, W.; Nicholson, P.; Al-Hussinee, L.; Subramaniam, K.; Waltzak, T.B. Genomic Characterization of Tilapia Lake Virus Isolates Recovered from Moribund Nile Tilapia (Oreochromis niloticus) on a Farm in the United States. Microbiol. Resour. Announc. 2020, 9, e01368-19.
- Eyngor, M.; Zamostiano, R.; Kembou Tsofack, J.E.; Berkowitz, A.; Bercovier, H.; Tinman, S.; Lev, M.; Hurvitz, A.; Galeotti, M.; Bacharach, E.; et al. Identification of a novel RNA virus lethal to tilapia. J. Clin. Microbiol. 2014, 52, 4137–4146.
- Behera, B.K.; Pradhan, P.K.; Swaminathan, T.R.; Sood, N.; Paria, P.; Das, A.; Verma, D.K.; Kumar, R.; Yadav, M.K.; Dev, A.K.; et al. Emergence of Tilapia Lake Virus associated with mortalities of farmed Nile Tilapia Oreochromis niloticus (Linnaeus 1758) in India. Aquaculture 2018, 484, 168–174.
- Dong, H.T.; Senapin, S.; Gangnonngiw, W.; Nguyen, V.V.; Rodkhum, C.; Debnath, P.P.; Delamare-Deboutteville, J.; Mohan, C.V. Experimental infection reveals transmission of tilapia lake virus (TiLV) from tilapia broodstock to their reproductive organs and fertilized eggs. Aquaculture 2020, 515, 734541.
- Nicholson, P.; Fathi, M.A.; Fischer, A.; Mohan, C.; Schieck, E.; Mishra, N.; Heinimann, A.; Frey, J.; Wieland, B.; Jores, J. Detection of Tilapia Lake Virus in Egyptian fish farms experiencing high mortalities in 2015. J. Fish Dis. 2017, 40, 1925–1928.
- Surachetpong, W.; Janetanakit, T.; Nonthabenjawan, N.; Tattiyapong, P.; Sirikanchana, K.; Amonsin, A. Outbreaks of Tilapia Lake Virus Infection, Thailand, 2015–2016. Emerg. Infect. Dis. 2017, 23, 1031–1033.
- Yamkasem, J.; Tattiyapong, P.; Kamlangdee, A.; Surachetpong, W. Evidence of potential vertical transmission of tilapia lake virus. J. Fish. Dis. 2019, 42, 1293–1300.
- Dong, H.; Siriroob, S.; Meemetta, W.; Sanimanawong, W.; Gangnonngiw, W.; Pirarat, N.; Khunrae, P.; Rattanarojpong, T.; Vanichviriyakit, R.; Senapin, S. Emergence of tilapia lake virus in Thailand and an alternative semi-nested RT-PCR for detection. Aquaculture 2017, 476, 111–118.
- Fathi, M.; Dickson, C.; Dickson, M.; Leschen, W.; Baily, J.; Muir, F.; Ulrich, K.; Weidmann, M. Identification of Tilapia Lake Virus in Egypt in Nile tilapia affected by ‘summer mortality’ syndrome. Aquaculture 2017, 473, 430–432.
- Ferguson, H.W.; Kabuusu, R.; Beltran, S.; Reyes, E.; Lince, L.A.; Del-Pozo, J. Syncytial hepatitis of farmed tilapia, Oreochromis niloticus (L.): A case report. J. Fish Dis. 2014, 37, 583–589.
- Koesharyani, I.; Gardenia, L.; Widowati, Z.; Khumaria, K.; Rustianti, D. Studi kasus infeksi tilapia lake virus (TiLV) pada ikan nila (Oreochromis niloticus). J. Ris. Akuakultur. 2018, 13, 85–92.
- Mugimba, K.K.; Chengula, A.A.; Wamala, S.; Mwega, E.D.; Kasanga, C.J.; Byarugaba, D.K.; Mdegela, R.H.; Tal, S.; Bornstein, B.; Dishon, A.; et al. Detection of tilapia lake virus (TiLV) infection by PCR in farmed and wild Nile tilapia (Oreochromis niloticus) from Lake Victoria. J. Fish. Dis. 2018, 41, 1181–1189.
- Mugimba, K.K.; Tal, S.; Dubey, S.; Mutoloki, S.; Dishon, A.; Evensen, Ø.; Munang’andu, H.M. Gray (Oreochromis niloticus x O. aureus) and Red (Oreochromis spp.) tilapia show equal susceptibility and proinflammatory cytokine responses to experimental Tilapia Lake Virus infection. Viruses 2019, 11, 893.
- Yamkasem, J.; Tattiyapong, P.; Gorgoglione, B.; Surachetpong, W. Uncovering the first occurrence of Tilapia parvovirus in Thailand in tilapia during co-infection with Tilapia tilapinevirus. Transbound. Emerg. Dis. 2021, in press.
- Jaemwimol, P.; Rawiwan, P.; Tattiyapong, P.; Saengnual, P.; Kamlangdee, A.; Surachetpong, W. Susceptibility of important warm water fish species to tilapia lake virus (TiLV) infection. Aquaculture 2018, 497, 462–468.
- Abdullah, A.; Ramly, R.; Ridzwan, M.S.M.; Sudirwan, F.; Abas, A.; Ahmad, K.; Murni, M.; Kua, B.C. First detection of tilapia lake virus (TiLV) in wild river carp (Barbonymus schwanenfeldii) at Timah Tasoh Lake, Malaysia. J. Fish. Dis. 2018, 41, 1459–1462.
- Pradhan, P.K.; Paria, A.; Yadav, M.K.; Verma, D.K.; Gupta, S.; Swaminathan, T.R.; Rathore, G.; Sood, N.; Lal, K.K. Susceptibility of Indian major carp Labeo rohita to tilapia lake virus. Aquaculture 2020, 515, 734567.
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; Available online: (accessed on 21 February 2021).
- FAO. Oreochromis Mossambicus (Peters, 1852). 2021. Available online: (accessed on 21 February 2021).
- Philippart, J.C.; Ruwet, J.C. Ecology and distribution of tilapias. In The Biology and Culture of Tilapias, In Proceedings of the ICLARM Conference of the ICLARM-International Center for Living Aquatic Resources Management, Manila, Philippines, 2–5 September 1980; ICLARM-International Center for Living Aquatic Resources Management: Manila, Philippines, 1982; pp. 15–59.
- Popma, T.J.; Lovshin, L.L. Worldwide Prospects for Commercial Production of Tilapia; Research and Development Series No. 41; Department of Fisheries and Allied Aquacultures Auburn University: Auburn, AL, USA, 1996; p. 23.
- Costa-Pierce, B.; Riedel, R. Fisheries ecology of the tilapias in the subtropical lakes of the United States. Tilapia Aquaculture in the Americas. J. Word. Aquac. Soc. 2000, 2, 1–20.
- Trewavas, E.; British, M. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis, and Danakilia; British Museum (Natural History): London, UK, 1983; Volume 1983.
- Uchida, K.; Kaneko, T.; Miyazaki, H.; Hasegawa, S.; Hirano, T. Excellent salinity tolerance of Mozambique tilapia (Oreochromis mossambicus): Elevated chloride cell activity in the branchial and opercular epithelia of the fish adapted to concentrated seawater. Zool. Sci. 2000, 17, 149–160.
- García-Ulloa, M.; Villa, R.L.; Martínez, M. Growth and feed utilization of the tilapia hybrid Oreochromis Mossambicus× O. Niloticus cultured at different salinities under controlled laboratory conditions. J. World. Aquac. Soc. 2001, 32, 117–121.
- King, M.; Sardella, B. The effects of acclimation temperature, salinity, and behavior on the thermal tolerance of Mozambique tilapia (Oreochromis mossambicus). J. Exp. Zool. A. Ecol. Integr. Physiol. J. 2017, 327, 417–422.
- Moorman, B.P.; Lerner, D.T.; Grau, E.G.; Seale, A.P. The effects of acute salinity challenges on osmoregulation in Mozambique tilapia reared in a tidally changing salinity. J. Exp. Biol. 2015, 218, 731–739.
- Baba, E.; Acar, Ü.; Öntaş, C.; Kesbiç, O.D.; Yilmaz, S. Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture 2016, 465, 13–18.
- Simbine, L.; Viana da Silva, J.; Hilsdorf, A.W.S. The genetic diversity of wild Oreochromis mossambicus populations from the Mozambique southern watersheds as evaluated by microsatellites. J. Appl. Ichthyol. 2014, 30, 272–280.
- D’Amato, M.E.; Esterhuyse, M.M.; Van der Waal, B.C.W.; Brink, D.; Volckaert, F.A.M. Hybridization and phylogeography of the Mozambique tilapia Oreochromis mossambicus in southern Africa evidenced by mitochondrial and microsatellite DNA genotyping. Conserv. Genet. 2007, 8, 475–488.
- Deines, A.M.; Bbole, I.; Katongo, C.; Feder, J.L.; Lodge, D.M. Hybridisation between native Oreochromis species and introduced Nile tilapia O. niloticus in the Kafue River, Zambia. Afr. J. Aquat. Sci. 2014, 39, 23–34.
- Firmat, C.; Alibert, P.; Losseau, M.; Baroiller, J.F.; Schliewen, U.K. Successive invasion-mediated interspecific hybridizations and population structure in the endangered cichlid Oreochromis mossambicus. PLoS ONE 2013, 8, e63880.
- Zengeya, T.A.; Booth, A.J.; Bastos, A.D.S.; Chimimba, C.T. Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environ. Biol. Fishes. 2011, 92, 479–489.
- Zengeya, T.A.; Robertson, M.P.; Booth, A.J.; Chimimba, C.T. Ecological niche modeling of the invasive potential of Nile tilapia Oreochromis niloticus in African river systems: Concerns and implications for the conservation of indigenous congenerics. Biol. Invasions 2013, 15, 1507–1521.
- Zengeya, T.A.; Robertson, M.P.; Booth, A.T.; Chimimba, C.T. A qualitative ecological risk assessment of the invasive Nile tilapia, Oreochromis niloticus in a sub-tropical African river system (Limpopo River, South Africa). Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 51–64.
- IUCN 2019. Oreochromis mossambicus (Errata Version Published in 2020), The IUCN Red List of Threatened Species 2019. 2019. Available online: (accessed on 21 February 2021).
- Nanthini, R.; Majeed, S.A.; Vimal, S.; Taju, G.; Sivakumar, S.; Kumer, S.S.; Pillai, D.; Sneha, K.G.; Rakesh, C.G.; Hameed, A.S.S. In vitro propagation of tilapia lake virus in cell lines developed from Oreochromis mossambicus. J. Fish Dis. 2019, 42, 1543–1552.
- Amal, M.N.A.; Koh, C.B.; Nurliyana, M.; Suhaiba, M.; Nor-Amalina, Z.; Santha, S.; Diyana-Nadhirah, K.P.; Yusof, M.T.; Ina-Salwany, M.Y.; Zamri-Saad, M.; et al. A case of natural co-infection of Tilapia Lake Virus and Aeromonas veronii in a Malaysian red hybrid tilapia (Oreochromis niloticus×O. mossambicus) farm experiencing high mortality. Aquaculture 2018, 485, 12–16.
This entry is offline, you can click here to edit this entry!
