Carpal tunnel syndrome (CTS), first described in 1863 by Sir James Paget, is the most common focal entrapment mononeuropathy. It is defined as a compression of the median nerve at the wrist associated with decreased function of the nerve at this level. There are several risk factors of CTS including genetic heredity, diabetes mellitus, thyroid disease, obesity, rheumatoid arthritis, and pregnancy.
- carpal tunnel release
- carpal tunnel syndrome
- median neuropathy
- electrodiagnostic study
1. Introduction
Carpal tunnel syndrome (CTS), first described in 1863 by Sir James Paget, is the most common focal entrapment mononeuropathy [1]. There are several risk factors of CTS including genetic heredity, diabetes mellitus, thyroid disease, obesity, rheumatoid arthritis, and pregnancy [2][3][4]. However, most cases of carpal tunnel syndrome are idiopathic [5]. CTS often affects persons of working age and may lead to absences from work and a marked decline in performance.
Primary diagnosis of CTS is based on characteristic clinical symptoms such as paresthesia (mainly numbness and tingling), pain at the wrist and hand and in severe cases weakness or atrophy of hand muscles. EDX studies include nerve conduction studies (NCSs) and electromyography (EMG). NCSs confirm CTS by detecting impaired median nerve conduction across the carpal tunnel, with normal conduction elsewhere. EMG assesses pathologic changes in the muscles innervated by the median nerve, typically the abductor pollicis brevis muscle [6].
Treatment options for CTS include wrist splinting, therapeutic ultrasound, local corticosteroid injections, and carpal tunnel release surgery [7]. Analysis of the costs of CTS treatment, including indirect non-healthcare costs such as loss of productivity caused by absence from work, led to the conclusion that established CTS is best treated with surgery [7]. Surgery has also been shown to be superior to non-operative treatment for patients with symptoms of CTS without changes due to denervation [8].
The results of electrodiagnostic studies have been found to be highly sensitive and specific for the diagnosis of CTS. EDX testing is performed by using generally accepted standardized techniques according to the American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) summary statement [6]. In patients suspected of CTS, the following EDX studies are recommended: median sensory or mixed nerve conduction study, median motor conduction study, needle examination of APB, ulnar or/and radial motor and sensory NCSs (in order to exclude a peripheral neuropathy), and needle electromyography of the limb muscles innervated by the C5toT1 roots (in order to exclude a cervical radiculopathy, brachial plexopathy, and a proximal median neuropathy) be performed as part of the examination of patients suspected of CTS [9]. In cases of severe CTS in which the median sensory potentials and the compound muscle action potential (CMAP) from abductor pollicis brevis (APB) muscle are absent, a lumbrical-interosseous distal motor latency (DML) comparison is performed [10].
Prolonged motor and sensory latencies of the median nerve and reduced sensory and motor conduction velocities in properly performed NCSs are accepted as diagnostic criteria for CTS. Many factors may influence the amplitude and latency of an individual nerve, giving a false-positive or false-negative result. Such factors include age, sex, finger diameter, concurrent systemic disease, obesity, and temperature. Applying a NCS as a relative comparison of two nerve segments, i.e., the median nerve and another nerve segment that does not travel through the carpal tunnel, could be useful.
2. Electrodiagnostic Studies in the Surgical Treatment of Carpal Tunnel Syndrome
A total of 2397 publications were identified by initial literature search. After additional records (n= 7) and removing duplications (n= 324), 2080 studies were included for initial assessment by title and/or abstract. Among those, 1956 publications were excluded after title/abstract screening, while 103 full texts were assessed for eligibility. Forty-five studies presenting irrelevant data and seventeen publications with reported incomplete data were excluded.
A total of 24 studies presented detailed pre- and postoperative data and extracted data of these publications were presented in Table S1 (supplement available online). The summary of Table 1and graphic presentation of the prognostic value of EDX according to confirmed significant correlation between preoperative EDX and recovery after surgery (Figure 1) are included below:
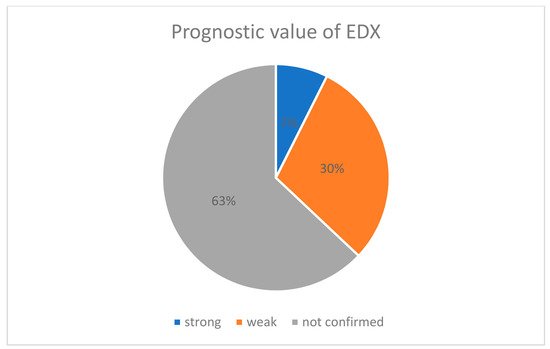
Figure 1. Percentage pie chart presenting prognostic value of EDX.
| Author | Number of EDX Tests | Number of Tests with Significant Prognostic Value | Grading (No = 0, Yes = 1) |
Prognostic Value (Strong = 2, Weak = 1, Not Confirmed = 0) |
|---|---|---|---|---|
| Schlagenhauff & Glasauer 1971 | 1 | 0 | 0 | 0 |
| Grudberg 1983 | 1 | 0 | 0 | 0 |
| Seror 1992 | 2 | 0 | 0 | 0 |
| Braun 1994 | 2 | 0 | 1 | 0 |
| Lange 1995 | 2 | 0 | 0 | 0 |
| Padua 1996 | 3 | 2 | 1 | 2 |
| Głowacki 1996 | 2 | 0 | 0 | 0 |
| Concannon 1997 | 1 | 0 | 1 | 0 |
| Padua 1997 | 3 | 3 | 1 | 2 |
| Choi 1998 | 2 | 0 | 1 | 0 |
| Nakamura 1999 | 4 | 0 | 1 | 0 |
| Dudley Porras 2000 | 4 | 2 | 1 | 1 |
| Mondelli 2001 | 5 | 1 | 1 | 1 |
| Finsen 2001 | 1 | 0 | 1 | 0 |
| Bland 2001 | 4 | 0 | 1 | 1 |
| Kouyoumdjian 2002 | 3 | 0 | 1 | 0 |
| Borisch 2003 | 2 | 0 | 0 | 0 |
| Schrijver 2005 | 3 | 0 | 0 | 0 |
| Tay 2006 | 2 | 0 | 1 | 0 |
| Malladi 2009 | 2 | 1 | 1 | 1 |
| Inukai 2012 | 5 | 1 | 1 | 1 |
| Tahririan 2012 | 3 | 0 | 1 | 0 |
| Beck 2013 | 1 | 0 | 1 | 0 |
| Fowler 2015 | 1 | 1 | 1 | 1 |
| Kronlage 2015 | 2 | 1 | 1 | 1 |
| DeKleemaeker 2017 | 4 | 0 | 0 | 0 |
| Rivlin 2018 | 1 | 1 | 1 | 1 |
| Rivlin 2018 | 1 | 1 | 1 | 1 |
| strong—significant confirmed prognostic value in all groups of patients | ||||
| weak—confirmed significant prognostic value in some groups | ||||
According to our results, the majority of publications did not confirm the prognostic value of EDX (only 2 of 28 articles). Padua et al. [11][12] revealed a strong correlation between neurophysiological severity of CTS and recovery, while 8 of 28 publications suggest a weak prognostic value of the EDX.
NOS scores of included publications are presented inTable 2. Mean NOS score (±SD) was 6.56 ± 1.34.
| Author | Selection | Comparability | Outcome |
|---|---|---|---|
| Schlagenhauff & Glasauer 1971 | 3 | 2 | 3 |
| Grudberg et al., 1983 | 3 | 1 | 2 |
| Luchetti et al., 1991 | 3 | 1 | 2 |
| Seror 1992 | 4 | 2 | 3 |
| Braun et al., 1994 | 2 | 1 | 1 |
| Lang et al., 1995 | 2 | 1 | 3 |
| Padua 1996 | 3 | 2 | 3 |
| Glowacki et al., 1996 | 3 | 2 | 3 |
| Concannon et al., 1997 | 3 | 1 | 2 |
| Higgs et al., 1997 | 3 | 1 | 3 |
| Padua et al., 1997 | 3 | 2 | 3 |
| Choi et al., 1998 | 3 | 2 | 3 |
| Nakamura et al., 1999 | 2 | 1 | 2 |
| Dudley Porras et al., 2000 | 2 | 1 | 1 |
| Mondelli et al., 2001 | 2 | 1 | 2 |
| Finsen et al., 2001 | 2 | 1 | 2 |
| Bland et al., 2001 | 2 | 1 | 1 |
| Uchiyama et al., 2002 | 3 | 1 | 3 |
| Kouyoumdjian et al., 2002 | 3 | 1 | 3 |
| Borisch et al., 2003 | 3 | 2 | 3 |
| Rotman et al., 2004 | 3 | 1 | 2 |
| Schrijver et al., 2005 | 3 | 2 | 2 |
| Tay et al., 2006 | 2 | 1 | 3 |
| Ginanneschi et al., 2008 | 2 | 1 | 3 |
| Malladi et al., 2009 | 3 | 1 | 3 |
| Inukai et al., 2012 | 3 | 1 | 3 |
| Tahririan et al., 2012 | 3 | 1 | 3 |
| Beck et al., 2013 | 3 | 2 | 3 |
| Fowler et al., 2015 | 3 | 1 | 3 |
| Kronlage et al., 2015 | 3 | 2 | 3 |
| De Kleermaeker et al., 2017 | 2 | 1 | 2 |
| Rivlin et al., 2018 | 3 | 2 | 2 |
| Mean ± SD | 2.72 ± 0.52 | 1.34 ± 0.48 | 2.50 ± 0.67 |
3. Discussion
This entry is adapted from the peer-reviewed paper 10.3390/jcm10122691
References
- Wang, L. Guiding treatment for carpal tunnel syndrome. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 751–760.
- Karpitskaya, Y.; Novak, C.B.; Mackinnon, S.E. Prevalence of smoking, obesity, diabetes mellitus, and thyroid disease in patients with carpal tunnel syndrome. Ann. Plast Surg. 2002, 48, 269–273.
- Palumbo, C.F.; Szabo, R.M.; Olmsted, S.L. The effects of hypothyroidism and thyroid replacement on the development of carpal tunnel syndrome. J. Hand Surg. Am. 2000, 25, 734–739.
- Stolp-Smith, K.A.; Pascoe, M.K.; Ogburn, P.L., Jr. Carpal tunnel syndrome in pregnancy: Frequency, severity, and prognosis. Arch. Phys. Med. Rehabil. 1998, 79, 1285–1287.
- Ashworth, N. Carpal tunnel. BMJ 2014, 349, g6437.
- Jablecki, C.K.; Andary, M.T.; Floeter, M.K.; Miller, R.G.; Quartly, C.A.; Vennix, M.J.; Wilson, J.R. Practice parameter: Electrodiagnostic studies in carpal tunnel syndrome. Neurology 2002, 58, 1589–1592.
- O’Connor, D.; Marshall, S.; Massy-Westropp, N.; Pitt, V. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2003, CD003219.
- Korthals-de Bos, I.B.; Gerritsen, A.A.; Van Tulder, M.W.; Rutten-van Mölken, M.P.; Adèr, H.J.; De Vet, H.C.; Bouter, L.M. Surgery is more cost-effective than splinting for carpal tunnel syndrome in the Netherlands: Results of an economic evaluation alongside a randomized controlled trial. BMC Musculoskelet Disord. 2006, 7, 86.
- Guidelines in electrodiagnostic medicine. American Association of Electrodiagnostic Medicine. Muscle Nerve 1992, 15, 229–253.
- Preston, D.C.; Logigian, E.L. Lumbrical and interossei recording in carpal tunnel syndrome. Muscle Nerve 1992, 15, 1253–1257.
- Padua, L.; Lomonaco, M.; Aulisa, L.; Tamburrelli, F.; Valente, E.M.; Padua, R.; Gregori, B.; Tonali, P. Surgical prognosis in carpal tunnel syndrome: Usefulness of a preoperative neurophysiological assessment. Acta Neurol. Scand. 1996, 94, 343–346.
- Padua, L.; Lo Monaco, M.; Padua, R.; Tamburrelli, F.; Gregori, B.; Tonali, P. Carpal tunnel syndrome: Neurophysiological results of surgery based on preoperative electrodiagnostic testing. J. Hand Surg. Br. 1997, 22, 599–601.
- Werner, R.A.; Andary, M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve 2011, 44, 597–607.
- De Kleermaeker, F.G.C.M.; Meulstee, J.; Claes, F.; Kasius, K.M.; Verhagen, W.I.M. Treatment outcome in patients with clinically defined carpal tunnel syndrome but normal electrodiagnostic test results: A randomized controlled trial. J. Neurol. 2017, 264, 2394–2400.
- Sonoo, M.; Menkes, D.L.; Bland, J.D.P.; Burke, D. Nerve conduction studies and EMG in carpal tunnel syndrome: Do they add value? Clin. Neurophysiol. Pract. 2018, 3, 78–88.
- Claes, F.; Kasius, K.M.; Meulstee, J.; Verhagen, W.I. Comparing a new ultrasound approach with electrodiagnostic studies to confirm clinically defined carpal tunnel syndrome: A prospective, blinded study. Am. J. Phys. Med. Rehabil. 2013, 92, 1005–1011.
- Mühlau, G.; Both, R.; Kunath, H. Carpal tunnel syndrome--course and prognosis. J. Neurol. 1984, 231, 83–86.
- Fowler, J.R.; Cipolli, W.; Hanson, T. A Comparison of Three Diagnostic Tests for Carpal Tunnel Syndrome Using Latent Class Analysis. J. Bone Jt. Surg. Am. 2015, 97, 1958–1961.
- Finestone, H.M.; Woodbury, G.M.; Collavini, T.; Marchuk, Y.; Maryniak, O. Severe carpal tunnel syndrome: Clinical and electrodiagnostic outcome of surgical and conservative treatment. Muscle Nerve 1996, 19, 237–239.
- Rivlin, M.; Kachooei, A.R.; Wang, M.L.; Ilyas, A.M. Electrodiagnostic Grade and Carpal Tunnel Release Outcomes: A Prospective Analysis. J. Hand Surg. Am. 2018, 43, 425–431.
- Basiri, K.; Katirji, B. Practical approach to electrodiagnosis of the carpal tunnel syndrome: A review. Adv. Biomed Res. 2015, 4, 50.
- Finsen, V.; Russwurm, H. Neurophysiology not required before surgery for typical carpal tunnel syndrome. J. Hand Surg. Br. 2001, 26, 61–64.
- Kortlever, J.T.P.; Becker, S.J.E.; Zhao, M.; Ring, D. Borderline Nerve Conduction Velocities for Median Neuropathy at the Carpal Tunnel. J. Hand Surg. Am. 2020, 45, 379–388.
- Miyaji, Y.; Kobayashi, M.; Oishi, C.; Mizoi, Y.; Tanaka, F.; Sonoo, M. A new method to define cutoff values in nerve conduction studies for carpal tunnel syndrome considering the presence of false-positive cases. Neurol. Sci. 2020, 41, 669–677.
- Inukai, T.; Uchida, K.; Kubota, C.; Takamura, T.; Nakajima, H.; Baba, H. Second lumbrical-interossei nerve test predicts clinical severity and surgical outcome of carpal tunnel syndrome. J. Clin. Neurosci. 2013, 20, 1224–1227.
- Inukai, T.; Uchida, K.; Kubota, C.; Takamura, T.; Nakajima, H.; Baba, H. Additional method for diagnosis of carpal tunnel syndrome: Value of the second lumbrical-interossei test (2L-INT). Hand Surg. 2013, 18, 49–52.
- Kasius, K.M.; Claes, F.; Verhagen, W.I.M.; Meulstee, J. Motor Nerve Conduction Tests in Carpal Tunnel Syndrome. Front. Neurol. 2019, 10, 149.
- Urits, I.; Gress, K.; Charipova, K.; Orhurhu, V.; Kaye, A.D.; Viswanath, O. Recent Advances in the Understanding and Management of Carpal Tunnel Syndrome: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 70.
- Sears, E.D.; Lu, Y.-T.; Wood, S.M.; Nasser, J.S.; Hayward, R.A.; Chung, K.C.; Kerr, E.A. Diagnostic Testing Requested Before Surgical Evaluation for Carpal Tunnel Syndrome. J. Hand Surg. Am. 2017, 42, 623–629.
- Sucher, B.M.; Schreiber, A.L. Carpal tunnel syndrome diagnosis. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 229–247.
- Colombo, J.; Shah, S. Utilization of Electrodiagnostic Testing for Carpal Tunnel Syndrome by General Practitioners Prior to Hand Surgery Consultation. Hand 2019, 14, 56–58.
- Ezquerra-Herrando, L.; Gómez-Vallejo, J.; Corella-Abenia, E.; Albareda-Albareda, J. Factores pronósticos en la cirugía del síndrome del túnel carpiano [Prognosis factors in carpal tunnel syndrome surgery]. Acta Ortop. Mex. 2014, 28, 160–163.
- American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. Available online: (accessed on 12 October 2017).
- Fowler, J.R.; Munsch, M.; Huang, Y.; Hagberg, W.C.; Imbriglia, J.E. Pre-operative electrodiagnostic testing predicts time to resolution of symptoms after carpal tunnel release. J. Hand Surg. Eur. Vol. 2016, 41, 137–142.
- Bland, J.D. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000, 23, 1280–1283.
- Mondelli, M.; Reale, F.; Padua, R.; Aprile, I.; Padua, L. Clinical and neurophysiological outcome of surgery in extreme carpal tunnel syndrome. Clin. Neurophysiol. 2001, 112, 1237–1242.
- Claes, F.; Kasius, K.M.; Meulstee, J.; Grotenhuis, J.A.; Verhagen, W.I. Treatment outcome in carpal tunnel syndrome: Does distribution of sensory symptoms matter? J. Neurol. Sci. 2014, 344, 143–148.
- Kronlage, S.C.; Menendez, M.E. The benefit of carpal tunnel release in patients with electrophysiologically moderate and severe disease. J. Hand Surg. Am. 2015, 40, 438–444.
- Bland, J.D. Do nerve conduction studies predict the outcome of carpal tunnel decompression? Muscle Nerve 2001, 24, 935–940.
- Iida, J.; Hirabayashi, H.; Nakase, H.; Sakaki, T. Carpal tunnel syndrome: Electrophysiological grading and surgical results by minimum incision open carpal tunnel release. Neurol. Med. Chir. 2008, 48, 554–559.
- Watchmaker, J.D.; Watchmaker, G.P. Independent Variables Affecting Outcome of Carpal Tunnel Release Surgery. Hand 2017, 13, 1558944717703739.
- Aghda, A.K.; Asheghan, M.; Amanollahi, A. Comparisons of electrophysiological and clinical findings between young and elderly patients with Carpal Tunnel Syndrome. Rev. Neurol. 2020, 176, 387–392.
- Braun, R.M.; Jackson, W.J. Electrical studies as a prognostic factor in the surgical treatment of carpal tunnel syndrome. J. Hand Surg. Am. 1994, 19, 893–900.
- Jordan, R.; Carter, T.; Cummins, C. A systematic review of the utility of electrodiagnostic testing in carpal tunnel syndrome. Br. J. Gen. Pract. 2002, 52, 670–673.
- Schrijver, H.M.; Gerritsen, A.A.M.; Strijers, R.L.M.; Uitdehaag, B.M.J.; Scholten, R.J.P.M.; De Vet, H.C.W.; Bouter, L.M. Correlating nerve conduction studies and clinical outcome measures on carpal tunnel syndrome: Lessons from a randomized controlled trial. J. Clin. Neurophysiol. 2005, 22, 216–221.
- Nathan, P.A.; Keniston, R.C.; Meadows, K.D. Electrical studies as a prognostic factor in the surgical treatment of carpal tunnel syndrome. J. Hand Surg. Am. 1996, 21, 526–527.
- Magnussen, M.J.; Morren, J. Electrodiagnostic Testing in the Diagnosis and Management of Carpal Tunnel Syndrome. Orthopedics 2017, 40, 263.
- Luchetti, R.; Schoenhuber, R.; Alfarano, M.; Montagna, G.; Pederzini, L.; Soragni, O. Neurophysiological assessment of the early phases of carpal tunnel syndrome with the inching technique before and during operation. J. Hand Surg. Br. 1991, 16, 415–419.
- Higgs, P.E.; Edwards, D.F.; Martin, D.S.; Weeks, P.M. Relation of preoperative nerve-conduction values to outcome in workers with surgically treated carpal tunnel syndrome. J. Hand Surg. Am. 1997, 22, 216–221.
- Rotman, M.B.; Enkvetchakul, B.V.; Megerian, J.T.; Gozani, S.N. Time course and predictors of median nerve conduction after carpal tunnel release. J. Hand Surg. Am. 2004, 29, 367–372.
- Glowacki, K.A.; Breen, C.J.; Sachar, K.; Weiss, A.P. Electrodiagnostic testing and carpal tunnel release outcome. J. Hand Surg. Am. 1996, 21, 117–121.
- Padua, L.; Aprile, I.; Monaco, M.L.; Padua, R.; Pasqualetti, P.; Nazzaro, M.; Tonali, P. Italian multicentre study of carpal tunnel syndrome: Clinical-neurophysiological picture and diagnostic pathway in 461 patients and differences between the populations enrolled in the northern, central and southern centres. Italian CTS Study Group. Ital. J. Neurol. Sci. 1999, 20, 309–313.
- Zhang, D.; Earp, B.E.; Blazar, P. Utility of ‘baseline’ electrodiagnostic studies for carpal tunnel release. J. Hand Surg. Eur. Vol. 2019, 44, 273–277.
- Claes, F.; Verhagen, W.I.; Meulstee, J. Current practice in the use of nerve conduction studies in carpal tunnel syndrome by surgeons in the netherlands. J. Hand Surg. Eur. Vol. 2007, 32, 663–667.
- Sangram, B.S.; Mayne, A.I.W.; Jariwala, A.C. Can we accurately predict nerve conduction study outcome using a carpal tunnel syndrome questionnaire? Surgeon 2019, 17, 156–159.
- Jansen, M.C.; Evers, S.; Slijper, H.P.; de Haas, K.P.; Smit, X.; Hovius, S.E.; Selles, R.W. Predicting Clinical Outcome After Surgical Treatment in Patients With Carpal Tunnel Syndrome. J. Hand Surg. Am. 2018, 43, 1098–1106.
- Lo, J.K.; Finestone, H.M.; Gilbert, K. Prospective evaluation of the clinical prediction of electrodiagnostic results in carpal tunnel syndrome. PM & R 2009, 1, 612–619.
- Gomes, I.; Becker, J.; Ehlers, J.A.; Nora, D.B. Prediction of the neurophysiological diagnosis of carpal tunnel syndrome from the demographic and clinical data. Clin. Neurophysiol. 2006, 117, 964–971.
- Fowler, J.R. Nerve Conduction Studies for Carpal Tunnel Syndrome: Gold Standard or Unnecessary Evil? Orthopedics 2017, 40, 141–142.
- Martić, V. Concordance of clinical and neurophysiologic diagnoses of carpal tunnel syndrome. Vojnosanit Pregl. 2015, 72, 247–250.
