Tormentic acid, also known as 2α,3β,19α-trihydroxyurs-2-en-28-oic acid (IUPAC Name: (1R,2R,4aS,6aR,6aS,6bR,8aR,10R,11R,12aR,14bS)-1,10,11-trihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid), is a pentacyclic triterpene. Its biological activity e.g. anti-inflammatory, antidiabetic, antihyperlipidemic, hepatoprotective, cardioprotective, neuroprotective, anti-cancer, anti-osteoarthritic, antinociceptive, antioxidative, anti-melanogenic, cytotoxic, antimicrobial, and antiparasitic has been confirmed in in vitro and in vivo studies. This molecule and its derivatives can be found in various plant species and families (e.g. Rosaceae, Lamiaceae, Myrtaceae, Oleaceae, Urticaceae, Boraginaceae), including edibles and herbs.
- tormentic acid
- triterpenes
- pentacyclic triterpene
- CAS 13850-16-3
- bioactivity
- plant metabolite
- tormentic acid derivatives
1. Introduction
2. Structure, Function, and Occurrence of TA
|
Plant Family |
Species and Organ Investigated |
Extraction Solvent |
Ref. |
|---|---|---|---|
|
Acanthaceae |
Rostellularia procumbens (L.) Nees [Justicia procumbens L.] Whole plant |
80% Ethanol |
[51] |
|
Aphloiaceae |
Aphloia theiformis (Vahl) Benn. Leaves |
Methanol |
[52] |
|
Aphloiaceae |
Aphloia theiformis (Vahl) Benn. Leaves |
70% Ethanol |
[53] |
|
Betulaceae |
Betula schmidtii Regel Twigs |
80% Methanol |
[15] |
|
Bignoniaceae |
Markhamia obtusifolia (Baker) Sprague Leaves |
Acetone |
[54] |
|
Bignoniaceae |
Markhamia platycalyx (Baker) Sprague [Markhamia lutea (Benth.) K.Schum.] Leaves |
95% Ethanol |
[55] |
|
Bignoniaceae |
Markhamia tomentosa (Benth) K. Schum ex Engl. Leaves |
Ethanol |
[19] |
|
Boraginaceae |
Anchusa italica Retz. [Anchusa azurea Mill.] Aerial parts |
75% Ethanol |
[18] |
|
Boraginaceae |
Arnebia euchroma (Royle) I.M.Johnst. Roots |
Methanol |
[56] |
|
Caprifoliaceae |
Cephalaria tuteliana Kuș & Göktürk Not specified |
Methanol |
[20] |
|
Caryophyllaceae |
Psammosilene tunicoides W.C. Wu & C. Y. Wu. Roots |
80% Ethanol |
[57] |
|
Compositae |
Kleinia pendula (Forssk.) DC. Fresh aerial parts |
Methanol |
[3] |
|
Ericaceae |
Rhododendron websterianum Rehder & E.H. Wilson Fruits |
95% Ethanol |
[21] |
|
Lamiaceae |
Hyptis capitata Jacq. Leaves and stems |
Methanol |
[58] |
|
Lamiaceae |
Isodon rubescens (Hemsl.) H.Hara Whole plant |
- |
[59] |
|
Lamiaceae |
Lavandula luisieri (Rozeira) Riv.-Mart. [Lavandula stoechas subsp. luisieri (Rozeira) Rozeira] Flowering plant |
Ethanol |
[41] |
|
Lamiaceae |
Leptohyptis macrostachys (L’H’erit.), Harley and J.F.B. Pastore (previously Hyptis macrostachys Benth.) Aerial parts |
95% Ethanol |
[60] |
|
Lamiaceae |
Ocimum gratissimum L. Aerial parts |
Methanol |
[61] |
|
Lamiaceae |
Perilla frutescens L. Britton Cell culture from leaves |
Methanol |
[45] |
|
Lamiaceae |
Perilla frutescens (L.) Britton var. acuta Kudo Fresh leaves |
Methanol |
[47] |
|
Lamiaceae |
Perilla frutescens (L.) Britton Leaves |
Ethanol |
|
|
Lamiaceae |
Platostoma rotundifolium (Briq.) A. J. Paton Aerial parts |
Ethyl acetate |
[62] |
|
Lamiaceae |
Salvia judaica Boiss. Aerial parts |
Ethanol |
[43] |
|
Lamiaceae |
Salvia miltiorrhiza Bunge Roots and aerial parts |
Ethanol |
[44] |
|
Leguminosae |
Campylotropis hirtella (Franch.) Schindl. Roots |
- |
[63] |
|
Malvaceae |
Triumfetta cordifolia A.Rich. Stems |
Methylene: methanol (1:1) |
[64] |
|
Myrtaceae |
Acca sellowiana (O.Berg) Burret Callus culture from fruit pulp |
Methanol |
[65] |
|
Myrtaceae |
Callistemon citrinus (Curtis) Skeels Leaves |
Dichloromethane: Methanol (50:50, v/v) Water: Ethanol (50:50, v/v) |
[66] |
|
Oleaceae |
Ligustrum robustum (Roxb.) Blume Not specified |
70% Methanol |
[22] |
|
Oleaceae |
Olea europaea L. Cell-suspension cultures (callus induced from leaf stalk) |
Methanol |
[23] |
|
Oleaceae |
Olea europaea L. (varieties Manzanilo, Picual, Koroneiki, and Coratina) Fruits |
Methanol |
[67] |
|
Oleaceae |
Osmanthus fragrans Lour Fruits |
Methanol |
[7] |
|
Polygonaceae |
Rumex japonicus Houtt. Stems |
80% Ethanol |
[24] |
|
Rosaceae |
Agrimonia pilosa Ledeb. Aerial parts |
80% Ethanol |
[29] |
|
Rosaceae |
Alchemilla faeroensis (J. Lange) Buser Aerial parts |
Ethanol |
[26] |
|
Rosaceae |
Cotoneaster simonsii hort. ex Baker Aerial parts (leaves and twigs) |
Chloroform |
[68] |
|
Rosaceae |
Crataegus pinnatifida Bunge Leaves |
80% Ethanol |
[30] |
|
Rosaceae |
Cydonia oblonga Mill. Seeds |
Methanol |
[34] |
|
Rosaceae |
Eriobotrya deflexa f. buisanesis [Eriobotrya deflexa (Hemsl.) Nakai.] Leaves |
Methanol |
[69] |
|
Rosaceae |
Eriobotrya fragrans Champ. ex Benth Leaves |
95% Ethanol |
[70] |
|
Rosaceae |
Eriobotrya japonica (Thunb) Lindl. Leaves |
80% Methanol |
[36] |
|
Rosaceae |
Eriobotrya japonica (Thunb.) Lindl. Leaves |
95% Ethanol |
|
|
Rosacae |
Eriobotrya japonica (Thunb.) Lindl Cell suspension culture (callus induced from leaves) |
Ethanol |
[37] |
|
Rosaceae |
Eriobotrya japonica (Thunb.) Lindl. Callus cultures induced from an axenic leaf |
Ethanol |
[38] |
|
Rosaceae |
Eriobotrya japonica (Thunb) Lindl. Cell suspension culture (obtained from immature embryos) |
95% Ethanol |
[39] |
|
Rosaceae |
Eriobotrya japonica (Thunb.) Lindl. Cell suspension culture (callus induced from leaves) |
95% Ethanol |
[4] |
|
Rosaceae |
Fragaria × ananassa Duch. var ‘Falandi’ Fresh fruit |
95% Ethanol |
[33] |
|
Rosaceae |
Fragaria × ananassa Duch. var ‘Hokouwase’ Green unripe fresh fruit |
Methanol |
[14] |
|
Rosaceae |
Geum japonicum auct. [Geum macrophyllum Willd.] Whole plant |
Methanol |
[71] |
|
Rosaceae |
Geum rivale L. Flowering aerial parts |
Chloroform: Methanol (9:1) |
[72] |
|
Rosaceae |
Geum urbanum L. Roots and aerial parts |
Methanol |
[28] |
|
Rosaceae |
Malus domestica Borkh varieties “Mela Rosa Marchigiana” and “Golden Delicious” Pulp callus culture |
Methanol |
[73] |
|
Rosaceae |
Margyricarpus setosus Ruiz & Pav. [Margyricarpus pinnatus (Lam.) Kuntze] Aerial parts |
Methanol |
[74] |
|
Rosaceae |
Potentilla anserina L. Roots |
- |
[75] |
|
Rosaceae |
Potentilla anserina L. Roots |
70% Ethanol |
[76] |
|
Rosaceae |
Potentilla chinensis Ser. Whole plant |
95% Ethanol |
[77] |
|
Rosaceae |
Potentilla fulgens [Potentilla lineata Trevir.] Roots |
Methanol |
[78] |
|
Rosaceae |
Poterium ancistroides Desf. [Sanguisorba ancistroides (Desf.) Ces.] Aerial parts |
Ethyl acetate |
[79] |
|
Rosaceae |
Poterium ancistroides Desf. [Sanguisorba ancistroides (Desf.) Ces.] Herb |
Methanol |
[80] |
|
Rosaceae |
Rosa nutkana C.Presl Fruits |
Methanol |
[81] |
|
Rosaceae |
Rosa roxburghii |
- |
[82] |
|
Rosaceae |
Rosa rugosa Thunb. Roots |
Methanol |
[32] |
|
Rosaceae |
Rubus chingii Hu Roots and rhizomes |
Ethanol |
[83] |
|
Rosaceae |
Rubus crataegifolius Bunge Leaves |
Methanol |
[27] |
|
Rosaceae |
Sanguisorba officinalis L. Root |
Cold water Hot water Methanol |
[84] |
|
Rosaceae |
Sarcopoterium spinosum (L.) Spach. Aerial parts |
- |
[85] |
|
Rubiaceae |
Knoxia valerianoides Thorel ex Pit. [Knoxia roxburghii subsp. brunonis (Wall. ex G.Don) R.Bhattacharjee & Deb] Roots |
Ethanol |
[86] |
|
Sapotaceae |
Tridesmostemon omphalocarpoides Engl. Wood and stem bark |
Dichloromethane: Methanol (1:1) |
[87] |
|
Saxifragaceae |
Tiarella polyphylla D. Don Whole plant |
Methanol |
[17] |
|
Staphyleaceae |
Euscaphis konishii Hayata [Euscaphis japonica (Thunb.) Kanitz] Twigs |
95% Ethanol |
[88] |
|
Urticaceae |
Cecropialyratiloba Miq. [Cecropia pachystachya Trécul.)] Roots |
Methanol |
[16] |
|
Urticaceae |
Cecropia pachystachya Trécul Roots, root bark, stem and stem bark |
Ethanol |
[25] |
|
Urticaceae |
Debregeasia salicifolia D. Don. [Debregeasia saeneb (Forssk.) Hepper & J.R.I.Wood] Stems |
Methanol |
[5] |
|
Urticaceae |
Myrianthus arboreus P.Beauv Stem bark |
Methylated ethyl acetate |
[50] |
|
Urticaceae |
Myrianthus arboreus P.Beauv Root wood |
Methylated spirit |
[48] |
|
Urticaceae |
Myrianthus arboreus P.Beauv Stems |
Chloroform |
[49] |
|
Urticaceae |
Myrianthus serratus (Trecul) Benth. Trunk wood |
Ethyl acetate |
[89] |
|
Urticaceae |
Pourouma guianensis Aubl. Leaves |
Methanol |
[90] |
|
Urticaceae |
Sarcochlamys pulcherrima (Roxb.) Gaudich. Aerial parts |
Methanol |
[91] |
|
Vochysiaceae |
Vochysia divergens Pohl. Stem bark |
Ethanol |
3. Pharmacological Activity of TA
|
Biological Activity |
Model |
Ref. |
|---|---|---|
|
Anti-inflammatory (anti-osteoarthritic): –decreasing the interleukin (IL)-1β-stimulated expression of MMP-3 and MMP-13; –inhibition of the IL-1β-induced expression of iNOS and COX-2, and the production of PGE2 and NO; inhibition of IL-1β-induced NF-κB activation |
In vitro Human Articular Chondrocyte Culture |
[97] |
|
Anti-inflammatory: –inhibition of nitric oxide (NO) and prostaglandin E 2 (PGE 2) production by inhibiting iNOS and COX-2 expression; –inhibition of LPS-stimulated production of TNF-α and IL-1β; –activation of LXRα (liver X receptor α) and inhibition of LPS-induced NF-κB activation |
In vitro BV2 microglial cells |
[98] |
|
Antioxidative and anti-inflammatory: –decreasing reactive oxygen species (ROS) generation; –inhibition of the expression of inducible nitric oxide synthase (iNOS) and NADPH oxidase (NOX); –decreasing the production of tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and IL-1β; –preventing phosphorylation of nuclear factor-κB (NF-κB) subunit p65 and degradation of NF-κB inhibitor α (IκBα) |
In vitro Rat vascular smooth muscle cells (RVSMCs); |
[99] |
|
Anti-inflammatory: –decreasing paw edema; –increasing the activities of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in liver tissue; –attenuating the level of thiobarbituric acid reactive substances (TBARS) in the edematous paw; –decreasing the nitric oxide (NO) levels at the serum level and diminishing the serum tumor necrosis factor (TNF-α); –decreasing the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) |
Ex vivo and in vivo RAW264.7 macrophages and λ-carrageenin-induced hind paw edema model in mice |
[37] |
|
Anti-inflammatory: –reducing the production of NO, prostaglandin E2 (PGE2), and tumor necrosis factor-α (TNF-α) induced by LPS; –suppressing the LPS-induced expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and TNF-α at the mRNA and protein levels; –decreasing DNA binding of nuclear factor kappa B(NF-kB) and nuclear translocation of the p65 and p50 subunits of NF-kB; –suppressing degradation and phosphorylation of inhibitor of kappa B-Alpha |
In vitro LPS stimulated RAW264.7 cells |
[100] |
|
Anti-inflammatory/antinociceptive (20–30 mg/kg) |
In vivo Writhing Assay; Hot-Plate Test; Carrageenan-Induced Edema in Sprague–Dawley Rats |
[32] |
|
Anti-inflammatory: –inhibition of the production of interleukin-6 and interleukin-8; –inhibition of TLR4 (Toll-like receptor 4) expression; –inhibition of activation of nuclear factor kappa B (NF-κB); –inhibition of activation of mitogen-activated protein kinases (MAPKs) |
In vitro LPS-stimulated human gingival fibroblasts (HGFs) |
[101] |
|
Anti-inflammatory: –inhibition of LPS-induced NO production |
In vitro |
[69] |
|
Anti-inflammatory: –inhibitory effect on IFN-γ secretion –inhibition of COX-1 and COX-2 –apoptosis-inducing effect |
In vitro LPS-stimulated Raw 264.7 macrophage |
[61] |
|
–Anti-inflammatory; –Potent inhibitory effect on Epstein-Barr virus early antigen (EBV-EA) activation; –Antitumor-promoting activity (strong) |
In vivo: –TPA-induced ear edema inflammation in mice; –two-stage carcinogenesis test of mouse tumor; In vitro EBV-EA activation experiment |
[42] |
|
–Cytotoxic activity against the HeLa cell line; –Antidiabetic activity –Inhibition of PTP1B (Protein tyrosine phosphate) |
In vitro |
[29] |
|
Cytotoxic to sensitive and multidrug resistant leukemia cell lines; Active toward a multidrug resistant (MDR) leukemia cell line overexpressing glycoprotein-P (P-gp) |
In vitro (anti-MDR activity in Lucena-1, a leukemia cell line that overexpresses P-gp and presents cross resistance to several unrelated cytotoxic drugs) |
[16] |
|
Cytotoxic |
In vitro HCT-8, A549, P-388, L-1210 tumor cell lines |
[58] |
|
–Cytotoxicity in human oral tumor cell lines: human salivary gland tumor and human oral squamous cell carcinoma –Inhibition of the activation of Epstein–Barr virus early antigen (EBV-EA) |
In vivo EBV genome-carrying lymphoblastoid cells In vitro human oral squamous cell carcinoma (HSC-2), human salivary gland tumor (HSG) |
[38] |
|
Antidiabetic and antihyperlipidemic: –Antihyperlipidemic: decreasing gene expressions of fatty acids, increasing the content of phosphorylated AMPK-α in liver and adipose tissue, inhibition of DGAT 1 expression, and decreasing the level of triglycerides in blood –Antidiabetic: down-regulation of phosphenolpyruvate carboxykinase (PEPCK), improving insulin sensitization (at 1.0 g/kg), and decreasing the expression of the hepatic and adipose 11-β-hydroxysteroid dehydroxygenase (11β-HSD1) gene |
In vivo high-fat fed C57BL/6J mice |
[4] |
|
Hypoglycemic: decreasing the blood glucose level (at 10 mg/kg) |
In vivo normoglycemic Wistar rats |
[79] |
|
Hypoglycemic effect (at 30 mg/kg): –decreasing glucose levels in normal rats; –increasing fasting plasma insulin levels Acute toxicity not observed (at 600 mg/kg, intraperitoneally) |
In vivo normoglycemic, hyperglycemic, and streptozotocin-induced diabetic Wistar rats |
[80] |
|
Hypoglycemic effect: –direct stimulation of insulin secretion by pancreatic islets of Langerhans |
In vitro pancreatic islets of Langerhans isolated from fed Wistar rats |
[102] |
|
Antidiabetic: –inhibition of alfa-glucosidase |
In vitro |
[78] |
|
Antidiabetic and antihyperlipidemic activity: –lowering blood glucose, triglycerides, free fatty acids, leptin levels; –decreasing the area of adipocytes and ballooning degeneration of hepatocytes; –reducing visceral fat mass, reducing hepatic triacylglycerol contents; –enhancing skeletal muscular Akt phosphorylation and increasing insulin sensitivity; –decreasing blood triglycerides by down-regulation of the hepatic sterol regulatory element binding protein-1c (SREBP-1c) and apolipoprotein C-III (apo C-III) and increasing the expression of peroxisome proliferator activated receptor (PPAR)-α |
In vivo C57BL/6J mice with induced type 2 diabetes and hyperlipidemia |
[103] |
|
Influencing the processes present in vasculoproliferative diseases (diseases related to vascular smooth muscle cell (VSMC) abnormal proliferation): –increasing apoptosis of serum-deprived A7r5 cells and inhibiting A7r5 cell proliferation; –rapid induction of significant modifications in the vascular smooth muscle cell (VSMC) phenotype; –inhibition of VSMC proliferation and VSMC cell death |
In vitro Clonal rat embryonic VSMCs (A7r5) and human umbilical vein endothelial cells (HUVEC) |
[93] |
|
Anti-melanogenesis effect (melanin synthesis inhibitory activity with less cytotoxicity) Antibacterial activity against Propionibacterium acnes Promotion of skin collagen synthesis |
In vitro Mouse melanoma cell line B16; Propionibacterium acnes (NBRC 107605) |
[104] |
|
Hepatoprotective (preventing fulminant hepatic failure): –blocking the NF-κB signaling pathway for anti-inflammatory response (alleviating the pro-inflammatory cytokines, e.g., TNF-α and NO/iNOS by inhibiting nuclear factor-κB activity); –inhibition of hepatic lipid peroxidation; –decreasing serum aminotransferase and total bilirubin activities; –attenuating hepatocellular apoptosis |
In vivo lipopolysaccharide/d-galactosamine-induced acute hepatic failure in male C57BL/6 mice |
[77] |
|
Hepatoprotective: –inhibition of the production of pro-inflammatory factors such as: tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and IL-6; –inhibition of inducible NO synthetase (iNOS) and cyclooxygenase-2 (COX-2); –inhibition of nuclear factor –κB (NF-κB) activation; –inhibition of the activation of mitogen-activated protein kinases (MAPKs); –retention of enzymes (essential for the antioxidative properties of liver): superoxidase dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) |
In vivo Acetaminophen-induced hepatotoxicity in male ICR mice |
[105] |
|
Protective effect against liver fibrosis: –inhibition of the activation of hepatic stellate cells; –reducing aspartate aminotransferase, alanine aminotransferase, and total bilirubin activity; –inhibition of expression of collagen type I and III; alleviation of collagen-based extracellular matrix deposition; –promoting cell apoptosis via blocking of the PI3K/Akt/mTOR and NF-κB signaling pathways |
In vitro Hepatic stellate cells (HSCs) stimulated with platelet-derived growth factor-BB |
[106] |
|
Cardioprotective (protective effects on hypoxia/reoxygenation (H/R)-induced cardiomyocyte injury) |
In vitro Neonatal rat cardiomyocytes subjected to hypoxia/reoxygenation (H/R) insult |
[18] |
|
Anti-hypoxic (protecting vascular endothelial cells against hypoxia-induced damage via the PI3K/AKT and ERK 1/2 signaling pathway) |
In vitro (EA.hy926 cells) |
[107] |
|
Antiproliferative: –causing apoptosis and G0/G1 phase arrest in cancer cell lines; –induction of cell cycle arrest via changing the cyclin D1 and cyclin-dependent kinase 4 mRNA expression levels; –down-regulation of the NF-kappa-B cell survival pathway and the expression level of phosphorylated ERK (extracellular signal-regulated kinase) |
In vitro Cancer cell lines: human hepatoma cells HepG-2 and Bel-7402, lung cancer cell A549, breast cancer cell MCF-7 Normal LO2 cell line |
[108] |
|
Antiproliferative |
In vitro |
[85] |
|
Anti-cancer (anti-hepatocellular carcinoma activity): –decreasing cell viability, colony formation, and cell migration; –induction of apoptosis; –changing the levels of caspase-3 and poly ADP-ribose polymerase expression |
In vitro Hepatocellular carcinoma cells (HepG2, Bel-7405, Sk-hep-1) |
[39] |
|
Anti-cancer: –induction of cell cycle arrest; –enhancement of ROS production; –targeting the mTOR/PI3K/AKT signaling pathway in cisplatin-resistant human cervical cancer cells |
In vitro Cisplatin-resistant human cervical cancer cells (HeLa cells) |
[95] |
|
Anti-osteoarthritic (inhibition of IL-1β-induced chondrocyte apoptosis by activation of the PI3K/Akt signaling pathway): –inhibition of IL-1β induced cytotoxicity and apoptosis in chondrocytes; –increasing B-cell lymphoma (Bcl)-2 expression; –decreasing capsase-3 activity and Bax expression; –increasing the expression of p-PI3K and p-Akt in IL-1β-induced chondrocytes |
In vitro IL-1β-treated human osteoarthritic chondrocytes |
[96] |
|
Antinociceptive (anti-allodynic) |
In vivo two models of chronic pain (neuropathic pain and inflammatory pain) in mice |
[92] |
|
Antibacterial |
In vitro |
[51] |
|
Antibacterial and antibiofilm effect: –inhibition of growth of P. aeruginosa; –depolarization of bacterial P. aeruginosa membrane; –inhibition of biofilm formation due to suppressed secretion of pyoverdine and suppressed secretion of protease and swarming motility of P. aeruginosa |
In vivo Mouse model of catheter infection for evaluation of antibiofilm activity and BALB/c mouse model for determination of in vivo toxicity In vitro P. aeruginosa cultures; murine macrophage cell line (RAW 264.7) for cytotoxicity assay |
[91] |
|
Antibacterial against S. aureus Antifungal against C. albicans |
In vitro |
[28] |
|
Antibacterial against S. aureus |
In vitro |
[81] |
|
Bacteriostatic against S. aureus: –inhibition of extracellular protease production resulting in inhibition of S. aureus growth |
In vitro |
[66] |
|
Antivirus: inhibition of virus HIV-1 protease |
In vitro |
[71] |
|
Insect antifeedant |
In vivo Spodoptera littoralis L6 larvae |
[41] |
|
Neuroprotective: –protecting against neurotoxicity (preventing neuronal loss); –blocking MPP+-induced apoptosis; –inhibiting intracellular accumulation of reactive oxygen species (ROS); –protecting from neuronal death through reversing the inhibition of the PI3-K/Akt/GSK3b pathway |
In vitro Parkinson’s disease cellular model: MPP+-induced neurotoxicity in human neuroblastoma SH-SY5Y cells |
[109] |
|
Neuroprotective: –decreasing amyloid plaque deposition; –reducing microglial activation and decreasing the secretion of pro-inflammatory factors; –suppressing the production of pro-inflammatory markers and the nuclear translocation of nuclear factor-κB (NF-κB); –reducing inhibited neurotoxicity and improving neuron survival |
In vivo Amyloid β precursor protein (APP)/presenilin 1 (PS1) transgenic mice In vitro BV2 microglia cells |
[94] |
4. Derivatives of Tormentic Acid
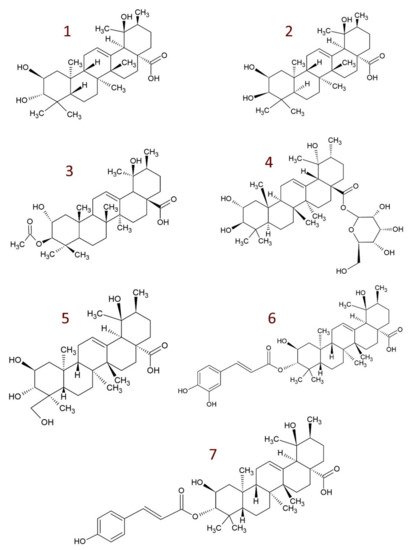
-
others, e.g., 6-methoxy-β-glucopyranosyl ester [112]; dihydrotormentic acid and methoxytormentic acid [110]; 3b-p-hydroxybenzoyloxytormentic acid [123]; (3R,19R)-methyl-3,19-dihydroxy-2-oxo-urs-12-en-28-carboxylate; (2R,19R)–methyl-2,19-dihydroxy-3-oxo-urs-12-en-28-carboxylate; (19R)-methyl-2,19-dihydroxyursa-3-oxo-1,12-dien-28-carboxylate; (2S,3R,19R)–methyl-2,3,19-trihydroxyurs-12-en-28-carboxylate; (2R,3R,19R)-2,3-bis(acetyloxy)-19-hydroxyurs-12-en-28-carboxylic acid; (2R,3R,19R)-2-acetyloxy-3,19-dihydroxyurs-12-en-28-carboxylic acid; (2R,3R,19R)-3-acetyloxy-2,19-dihydroxyurs-12-en-28-carboxylic acid; (3R,19R)–methyl-3-acetyloxy-19-hydroxy-2-oxo-urs-12-en-28-carboxylate; (2R,19R)-methyl-2-acetyloxy-19-hydroxy-3-oxo-urs-12-en-28-carboxylate; (2R,3R,19R)–methyl-2,3-bis(chloroacetyloxy)-19-hydroxy-urs-12-en-28-carboxylate; (2R,3R,19R)–methyl-2-chloroacetyloxy-3,19-dihydroxyurs-12-en-28-carboxylate; (2R,3R,19R)–methyl-3-chloroacetyloxy-2,19-dihydroxyurs-12-en-28-carboxylate [9].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26133797
