Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Pseudoephedrine (PSE) is a drug with a long history of medical use; it is helpful in treating symptoms of the common cold and flu, sinusitis, asthma, and bronchitis.
- pseudoephedrine
- sympathomimetic
1. Introduction
Pseudoephedrine (PSE) and ephedrine (E) are alkaloids derived from various species of Ephedra spp. of the Ephedraceae family. The most common source of their extraction is Ephedra sinica, also known as Ma Huang. The history of the use of Ephedra products in medicine is very long; they have been used in China for over 5000 years and in the Middle East for over 2000 years in the treatment of bronchial asthma, fever, coughs and colds, hay fever, oedema, bronchitis, urticaria, chronic hypotension, and rheumatism. Nowadays, they are also used as stimulants, the so-called energisers, and as agents reducing appetite, body weight and increasing energy consumption.
2. Mechanism of Action
Pseudoephedrine is a sympathomimetic with a mixed mechanism of action, direct and indirect. It indirectly stimulates alpha-adrenergic receptors, causing the release of endogenous norepinephrine (NE) from the granularity of neurons, while it directly stimulates beta-adrenergic receptors [1][2][3].
It has an effect similar to ephedrine, but slightly weaker, and has a lower ability to induce tachycardia and increase systolic blood pressure. Its central effect is weaker than that of amphetamine, and its peripheral effect is similar to that of epinephrine [4]. The mechanism of pseudoephedrine action is shown graphically in Figure 1.
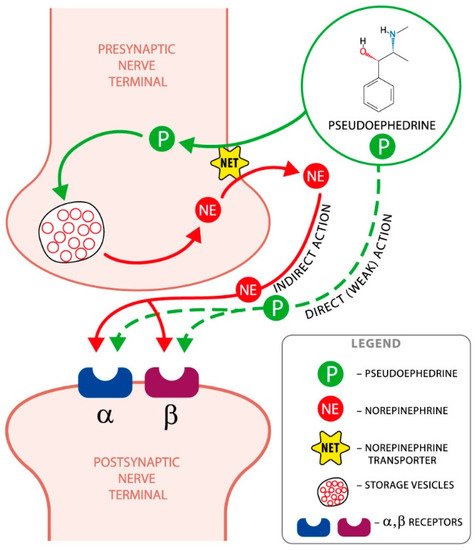
Figure 1. The mechanism of pseudoephedrine action. The principal mechanism by which pseudoephedrine achieves its effects is by displacing the norepinephrine (noradrenaline) from the storage vesicles in the presynaptic neurons; then, it is released into the neuronal synapse and becomes available to activate the alpha and beta postsynaptic adrenergic receptors.
3. Pharmacokinetics
Unlike epinephrine and norepinephrine, pseudoephedrine is active after oral administration and is easily absorbed from the gastrointestinal tract. The onset of action occurs after 30 min and after 1–4 h the drug reaches its maximum concentration in the blood. When using the extended-release formulation, this time is twice as long. PSE is mainly excreted unchanged in the urine (43–96%); only a small amount, approximately 1–6%, is metabolised in the liver by N-demethylation to the active metabolite norpseudoephedrine (cathine). The time the drug remains in the body depends on the pH of the urine; the value of the biological half-life (t0.5) decreases when the urine is acidic, and increases when the urine is alkaline [5][6][7][8][9][10][11]. Selected pharmacokinetic properties of pseudoephedrine are presented in Table 1.
Table 1. Pseudoephedrine pharmacokinetics.
| Pharmacokinetic Parameters of Pseudoephedrine | |
|---|---|
| Onset of action | 30 min |
| Time to reach Cmax | 1–4 h |
| Time to reach Cmax after administration of the extended-release formulation | 2–6 h |
| Duration of action | 4–12 h |
| Distribution coefficient | 2.64–3.51 l/kg |
| Biological half-life | 3–16 h |
| Renal clearance | 0.44–0.46 l/h/kg, 7.3–7.7 mL/min/kg |
4. Consequences of Pseudoephedrine Use
The drug reduces congestion of the upper respiratory tract mucosa, especially in the nose and paranasal sinuses (after oral administration), which in turn reduces the swelling, the amount of secretions and clears the nose. The sympathomimetic effect of pseudoephedrine may also improve the patency of the Eustachian tube and equalise the pressure in the middle ear during changes in atmospheric pressure while diving or flying by plane. The administration of 120 mg of pseudoephedrine to an adult at least 30 min before a flight may reduce earache. However, no similar effect has been observed in children. Pseudoephedrine is also effective in cases of urinary incontinence [4][12].
Similarly to other sympathomimetics, PSE stimulates the sympathetic system to fight-or-flight reactions—speeds up breathing, increases blood pressure, accelerates heart rate, narrows peripheral blood vessels, causes bronchodilatation, increases blood glucose levels, stimulates the CNS, as well as giving a sense of an energy surge and improving mood [4][13].
5. Clinical Use and Contraindications
Pseudoephedrine is recommended for the symptomatic treatment of obstruction in the nasal cavity, paranasal sinuses and the Eustachian tube. Other indications include vasomotor rhinitis and adjunctive therapy in allergic rhinitis and otitis media [4][10][11].
Contraindications to pseudoephedrine use are hypersensitivity to the drug, cardiovascular diseases (hypertension and coronary artery disease), impaired function of organs responsible for elimination of the drug (severe liver dysfunction, moderate or severe renal dysfunction), hyperthyroidism, narrow-angle glaucoma, benign prostatic hyperplasia, diabetes mellitus, mental agitation and treatment with monoamine oxidase inhibitors (MAO inhibitors) currently or in the last two weeks. Contraindications also include physiological conditions such as pregnancy and lactation, and age under 2 years. The extended-release form of the drug should not be used until the age of 12 years [10][11].
Although there are no reports of pseudoephedrine disturbing psychophysical performance, people driving motor vehicles should exercise caution and not use doses higher than recommended.
Studies evaluating the effect of this drug on daytime sleepiness and fatigue in patients suffering from perennial allergic rhinitis showed no positive or negative effect compared to placebo [14].
6. Adverse Reactions
Pharmacotherapy is inevitably associated with the risk of drug-related complications; the most controversial is the effect of pseudoephedrine on blood pressure and its consequences. Some literature data suggest that oral sympathomimetic drugs may dangerously increase blood pressure, while others reassure that the danger is exaggerated. One meta-analysis of randomised controlled trials showed that PSE at the recommended doses had no effect on systolic and diastolic blood pressure in healthy or controlled hypertensive patients. Systolic blood pressure increases by an average of 1 mm Hg and the heart rate increases by three beats per minute. Only about 3% of the analysed patients had pressures above 140/90 mm Hg [15]. During the use of pseudoephedrine preparations, reported cases include an acute coronary syndrome in a patient who performed hard physical work and smoked for 30 years; a myocardial infarction, hypertensive crisis and NSTEMI (Non-ST-Segment Elevation Myocardial Infarction) without ST segment elevation after taking the drug in the extended-release form by an 87-year-old man with history of mild dementia, glaucoma and atrial fibrillation; as well as an increased blood pressure of 220/140 mm Hg, hyperglycaemia, haemorrhagic stroke, strong, reversible spasm of blood vessels and tachycardia in a driver working at night, a nicotine addict and concomitant drug user for more than 20 years [7][15][16][17][18]. The effect of pseudoephedrine may induce non-convulsive epileptic states in predisposed individuals with pre-existing neurological disorders [7][19]. The risk of complications increases in patients with impaired renal and hepatic function. Unusual behaviour and myoclonic convulsions were observed in a 64-year-old man suffering from renal failure, who took 240 mg of PSE daily to treat rhinitis [7]. Severe agitation and disorientation can be expected in patients with phenylketonuria due to a disturbed metabolism of catecholamines [4]. Adverse effects of PSE can occur with both oral and intranasal administration after a single dose or after prolonged (5 days) treatment, without affecting the dose and irrespective of the vascular condition and age. A 2003 French study analysed adverse events with intranasal decongestants reported to regional pharmacovigilance centres by healthcare professionals. There were 22 episodes of arterial hypertension, 15 cases of convulsions and 4 cases of stroke after oral administration of drugs containing pseudoephedrine [20]. It can also induce ischemic colitis when used for as little as 3 days or up to 2 years, in a dose range of 60 to 900 mg/day [21]. Less common adverse effects are skin reactions—cases of scarlet fever-like rash, erythematous spots, skin exfoliation of the palms and soles of the feet, and Baboon syndrome, clinically manifested by a rash mainly on the buttocks and within the larger folds of skin, have been reported [22][23]. When used in therapeutic doses, PSE may be responsible, especially in children, for the occurrence of pain and dizziness, increased heart rate, excessive agitation, insomnia and hallucinations [4]. “Parasitic” hallucinoses (attacking spiders and insects) have been observed in children after taking an OTC (over-the-counter) drug containing pseudoephedrine and triprolidine to treat inflammation of the nasal mucosa [4][7]. In 2007 Wingert et al. detected pseudoephedrine in a postmortem analysis of 13 unexpected deaths of children under 2 years of age taking cold medications in the Philadelphia region. Similar observations were made in 2008 by Rimsza and Newberry, who reviewed case files of unexpected deaths of children taking cold medications. PSE preparations should not be used in patients before the age of 12, and according to the French Society of Otorhinolaryngology, until the age of 15 [20]. On the pharmaceutical market, however, there are preparations allowing their administration to younger patients, e.g., from 7 years of age. The addictive potential of PSE is confirmed by the case of a 37-year-old woman who abused it for its euphoric effect, increasing the doses over five years, using 3000–4500 mg daily. Sudden discontinuation of the drug resulted in depressed mood, visual hallucinations and a feeling of fatigue [7].
This entry is adapted from the peer-reviewed paper 10.3390/ijms22105146
References
- Chiarugi, A.; Camaioni, A. Update on the pathophysiology and treatment of rhinogenic headache: Focus on the ibuprofen/pseudoephedrine combination. Acta Otorinol. Ital. 2019, 39, 22–27.
- Trinh, K.V.; Kim, J.; Ritsma, A. Effect of pseudoephedrine in sport: A systemic review. BMJ Open Sport Exerc. Med. 2015, 1, e000066.
- Gheorghiev, M.D.; Hosseini, F.; Moran, J.; Cooper, C.E. Effects of pseudoephedrine on parameters affecting exercise performance: A meta-analysis. Sports Med. Open 2018, 4, 44.
- Piątek, A.; Koziarska-Rościszewska, M.; Zawilska, J.B. Recreational use of over-the counter drugs: The doping of the brain. Alcohol. Drug Addict. 2015, 28, 65–77.
- Wellington, K.; Jarvis, B. Cetirizine/Pseudoephedrine. Drugs 2001, 61, 2231–2240.
- Wang, L.; Feng, F.; Wang, X.Q.; Zhu, L. Influences of urinary pH on the pharmacokinetics of three amphetamine-type stimulants using a new high-performance liquid chromatographic method. J. Pharm. Sci. 2009, 98, 728–738.
- Pawlaczyk, M.; Korzeniowska, K.; Jabłecka, A. Safety and efficacy of pseudoephedrine. Farm. WSP 2017, 10, 67–71.
- Kale, P. Pharmacokinetics and bioavailability of single dose ibuprofen and pseudoephedrine alone or in combination: A randomized three-period, cross-over trial in healthy Indian volunteers. Front. Pharmacol. 2014, 5.
- Flanagan, S.; Minassian, S.L.; Prokocimer, P. Pharmacokinetics of Tedizolid and Pseudoephedrine Administered Alone or in Combination in Healthy Volunteers. J. Clin. Med. 2018, 7, 150.
- Pseudoephedrine. Available online: (accessed on 21 March 2021).
- Summary of Product Characteristics Sudafed. Available online: (accessed on 21 March 2021).
- What is the Role of Alpha Agonists in Urinary Incontinence Treatment? Available online: (accessed on 20 March 2021).
- Barroso, O.; Goudreault, D.; Banus, M.C.; Ayotte, C.; Mazzoni, I.; Boghosian, T.; Rabin, O. Determination of urinary concentrations of pseudoephedrine and cathine after therapeutic administration of pseudoephedrine-containing medications to healthy subjects: Implications for doping control analysis of these stimulants banned in sport. Drug Test. Analysis 2011, 4, 320–329.
- Sherkat, A.A.; Sardana, N.; Safaee, S.B.; Lehman, E.B.; Craig, T.J. The role of pseudoephedrine on a daytime somnolence in patients suffering from perennial allergic rhinitis (PAR). Ann. Allergy Asthma Immunol. 2011, 106, 97–102.
- Salerno, S.M.; Jackson, J.L.; Berbano, E.P. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch. Intern. Med. 2009, 6, 1686–1694.
- Serhat, A.; Metehan, O. Acute coronary syndrome presenting after pseudoephedrine use and regression with beta-blocker therapy. Can. J. Cardiol. 2008, 24, e86–e88.
- Celic, A. ST elevation myocardial infarction presenting after use of pseudoephedrine. Cardiovasc. Toxicol. 2009, 9, 103–104.
- Bharatula, A.; New, P.W. Cough mixture dependence and stroke: Implications for pseudoephedrine regulation. Med. J. Aust. 2011, 194, 427.
- Ismailogullari, S.; Yetkin, M.F.; Erdogan, F.; Delibas, E.; Aksu, M.; Ersoy, A.O. Letter to the Editor, Pseudoephedrine-induced nonconvulsive status epilepticus. Epilepsy Behav. 2011, 20, 739–740.
- Laccourreye, O.; Werner, A.; Giroud, J.P.; Couloigner, V.; Bonfils, P.; Bondon-Guitton, E. Benefits, limits and danger of ephedrine and pseudoephedrine as nasal decongestants. Europ. Annals Otorhinolaatyngol. Head Neck Dis. 2015, 132, 31–34.
- Aziz, M.; Pervez, A.; Fatima, R.; Bansal, A. Case Report Pseudoephedrine Induced Ischemic Colitis: A Case Report and Review of Literature. Case Rep. Gastrointest. Med. 2018, 2018.
- Özkaya, E.; Elinç-Aslan, M.S. Pseudoephedrine May Cause “Pigmenting” Fixed Drug Eruption. Dermatitis 2011, 22, 7–9.
- Ozdemir, H.; Celik, N.G.; Tapisis, A.; Akay, B.N.; Ciftci, E.; Ince, E.; Dogru, U. Baboon syndrom induced by oral antitussive-decongestant agent in a child. Turk. J. Pediatr. 2010, 52, 659–661.
This entry is offline, you can click here to edit this entry!
