Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
Poly (methyl methacrylate) (PMMA) is a thermoplastic synthetic polymer, which displays superior characteristics such as transparency, good tensile strength, and processability.
- poly (methyl methacrylate) (PMMA)
- atomic layer deposition (ALD)
1. Introduction
Poly (methyl methacrylate) (PMMA) is a transparent thermoplastic synthesized by emulsion polymerization, solution polymerization, and bulk polymerization from the MMA monomer [1]. This acrylate has high resistance to sunlight exposure and good optical properties, widely used to substitute and enhance the glass performance [2]. This polymeric compound is attractive; hence is stable, affordable, has been explored in multiple structural and forms—like sheets, films, tubular, even spherical composites—from nanotechnology to upper metrics/scales with variety being applied in all kinds of industries [3][4]. One of the main advantages of PMMA is that it contains less potentially harmful subunits from the synthesis, like bisphenol-A, commonly found in other types of polymers such as polycarbonates, polysulfones, and epoxy resins [5]. It is a superior polymeric material for analytical separation, sensing [6], biomedical and medical applications due to biocompatibility [7][8], and is used for electrolysis [9], polymer conductivity, viscosity measurements [10][11], solar nano/micro concentrator lens for solar cells [12][13][14]. In practice, the PMMA surface properties can be tailored by surface modification through graft copolymerization [15] or by the incorporation of a surfactant into the polymer matrix [16].
2. ALD Coatings on PMMA Aided by Seed Layer
The literature above presented demonstrated that PMMA is a viable polymer material for ALD coating process. However, the requirement of a low deposition temperature due to its low Tg raises another limitation: the known ALD precursors for thin film formation at low temperatures are very limited. A solution can be the use of seed layers.
Wilson et al. reported on W deposition on polymers by ALD and their results showed that W nucleation was enhanced by a few previous cycles of Al2O3 by ALD. In this case, Al2O3 acts as a seed layer of nucleation on a variety of spin-coated polymers such as PMMA, polyvinyl chloride (PVC), polystyrene (PS), polypropylene (PP), and polycarbonate (PC). A growth per cycle (GPC) of 3.9 Å for W ALD at 80 °C has been attained, as shown in Figure 1 [17].
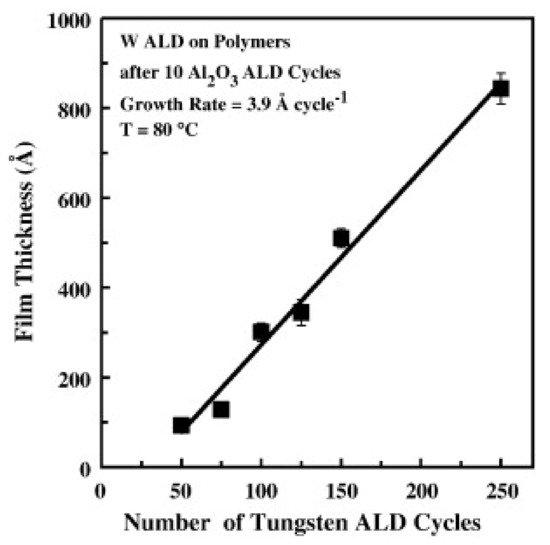
Figure 1. Profilometry measurements of W ALD film thickness on different polymers vs. the number of W ALD cycles. Al2O3 ALD was used as a seed layer (10 ALD cycles) (reprinted from [17], Copyright (2008), with permission from Elsevier).
Minton et al. followed the same strategy in terms of using Al2O3 ALD seed layer prior to TiO2 ALD, because TiO2 did not nucleate well on the PMMA surface. Their results showed that the uncoated PMMA lost a considerable part of its mass, when exposed in vacuum to UV radiation and the bilayers formed with 20 cycles of Al2O3 and 100 or 200 cycles of TiO2 were efficient in preserving PMMA (Figure 2) [18].
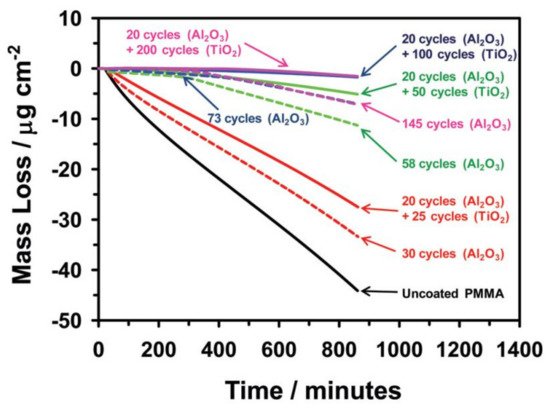
Figure 2. Mass loss of uncoated and coated PMMA with Al2O3 and Al2O3/TiO2 when exposed to vacuum UV radiation over time (reprinted with permission from [18], Copyright (2010) American Chemical Society).
Kemell et al. also explored the ability of ALD coatings of Al2O3 and TiO2 on PMMA films, among other polymer films, like polyether ether ketone (PEEK), polytetrafluoroethylene (PTFE), and ethylene tetrafluoroethylene (ETFE) [19]. The deposition temperature range was from 80 to 250 °C, enabling the synthesis of amorphous and crystalline metal oxides. Firstly, amorphous Al2O3 was deposited at 150–250 °C followed by TiO2 deposited at 100 °C. For the case of TiO2 deposition on Al2O3-coated PMMA at 250 °C, polycrystalline anatase TiO2 films were obtained, as shown in Figure 3 These results highlight the importance of Al2O3 seed layer, or interlayer, prior to TiO2. In fact, TiO2 deposition on bare PMMA was attempted also at 250 °C, but virtually no growth was observed, revealing that TiO2 does not nucleate well on PMMA. Interestingly, the authors did not point out any constraints of the polymer’s thermal stability when deposition at 250 °C, in particular regarding PMMA stability.
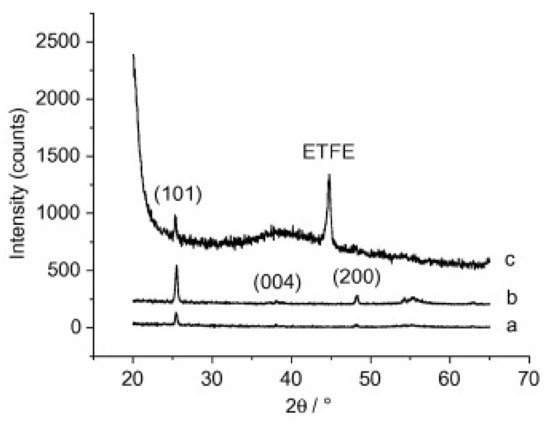
Figure 3. XRD patterns of anatase TiO2 films in substrates made of (a) Si at 200 °C, (b) PMMA at 250 °C, and (c) ETFE ate 200 °C. Si substrate was used as a reference, for comparison purpose (reprinted from [19] Copyright (2008), with permission from Elsevier).
An identical approach was performed by Napari et al. Authors reported on the influence of the deposition of an Al2O3 seed layer to the ZnO film growth, morphology, and crystallinity, on PMMA commercial plates and spin-coated PMMA films on Si substrate. The Al2O3 seed layer provides a pathway for blocking the DEZ precursor into the PMMA subsurface and improves the ZnO growth with some degree of hexagonal crystal orientation at low deposition temperature viz 35 °. The ZnO surface wetting properties were altered upon UV illumination. As a consequence, this photoinduced changes on the wettability find applications in microfluidics, where thin functional coatings on patterned polymer platforms can be used to manipulate the fluid flows [20]. This work is a promising alternative for lab-on-a-chip technologies development and microfluidics platforms.
In brief, the seed layer or interlayer approach on polymers can be seen as an in-situ two-step ALD process, consisting of the deposition of a few nanometer seed-like layer, at a lower temperature step, followed by a second process for the more refractory metal oxides. The choice of Al2O3 ALD from TMA and H2O precursors is a viable pathway to seeding layer on polymers because Al2O3 ALD can be conducted at temperatures as low as 35 °C, conjugated with the TMA positive characteristics like its high volatility and reactivity towards different co-reactants at low temperatures. Based on the above studies, the majority of published work for ALD on polymers addresses Al2O3 ALD from TMA and H2O cycles, stressing out the versatility of this ALD process either as coating and/or seed layer, being also a method to study the influence of the polymer substrate properties on the nucleation and growth of metal oxides. Similar to Al2O3, ZnO ALD can also be performed on polymers by taking advantage of the DEZ high volatility and reactivity at low temperatures. It is clear that the modification of a polymer substrate that shows high reactivity towards a given ALD process is crucial to ensure high-density nucleation towards a homogeneous and uniform thin film.
This entry is adapted from the peer-reviewed paper 10.3390/polym13081346
References
- Goseki, R.; Ishizone, T. Poly(methyl methacrylate) (PMMA). Encycl. Polym. Nanomater. 2014, 1–11.
- Hu, L.; Yang, Z.; Zhang, X.; Liu, Z.; Xia, P.; Deng, K.; Gong, L.; Jiang, L.; Zhang, H. Fabrication and evaluation of dual function PMMA/nano-carbon composite particles for UV curable anti-glare coating. Prog. Org. Coat. 2016, 101, 81–89.
- Van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Nijenhuis, K., Ed.; Elsevier Publications: Amsterdam, The Netherlands, 2000; ISBN 9780080548197.
- Shah, J.J.; Geist, J.; Locascio, L.E.; Gaitan, M.; Rao, M.V.; Vreeland, W.N. Surface modification of poly(methyl methacrylate) for improved adsorption of wall coating polymers for microchip electrophoresis. Electrophoresis 2006, 27, 3788–3796.
- Peters, E.N. Thermoplastics, Thermosets, and Elastomers—Descriptions and Properties. In Mechanical Engineers’ Handbook; Kutz, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; SABIC: Selkirk, NY, USA, 2015; pp. 1–48. ISBN 9781118985960.
- Sharif, M.M.; Johari, M.A.M.; Al Noman, A.; Abdul Khudus, M.I.M.; Harun, S.W. PMMA microfiber and Microball Resonator for fomaldehyde liquid sensing. Sens. Actuators A Phys. 2020, 304, 111828.
- Al-Nemrawi, N.K.; Marques, J.; Tavares, C.J.; Oweis, R.J.; Al-Fandi, M.G. Synthesis and characterization of photocatalytic polyurethane and poly(methyl methacrylate) microcapsules for the controlled release of methotrexate. Drug Dev. Ind. Pharm. 2018, 44, 2083–2088.
- Alves Batista, F.; Brena Cunha Fontele, S.; Beserra Santos, L.K.; Alves Filgueiras, L.; Quaresma Nascimento, S.; de Castro e Sousa, J.M.; Ramos Gonçalves, J.C.; Nogueira Mendes, A. Synthesis, characterization of α-terpineol-loaded PMMA nanoparticles as proposed of therapy for melanoma. Mater. Today Commun. 2020, 22.
- Li, X.; Li, D.; Liu, X.; Chang, H. Ultra-monodisperse droplet formation using PMMA microchannels integrated with low-pulsation electrolysis micropumps. Sens. Actuators B Chem. 2016, 229, 466–475.
- Deepa, M.; Sharma, N.; Agnihotry, S.A.; Singh, S.; Lal, T.; Chandra, R. Conductivity and viscosity of liquid and gel electrolytes based on LiClO4, LiN(CF3SO2)2 and PMMA. Solid State Ionics 2002, 152–153, 253–258.
- Devikala, S.; Kamaraj, P.; Arthanareeswari, M. AC conductivity studies of PMMA/TiO2 composites. Mater. Today Proc. 2018, 5, 8678–8682.
- Ko, Y.; Kim, Y.; Lee, C.; Kim, Y.; Jun, Y. Poly(methyl methacrylate) embedded perovskite films for improving solar cell performance. Synth. Met. 2019, 249, 47–51.
- Alves, M.; Pérez-Rodríguez, A.; Dale, P.J.; Domínguez, C.; Sadewasser, S. Thin-film micro-concentrator solar cells. J. Phys. Energy 2019, 2, 012001.
- Loffredo, F.; Villani, F.; Cancro, C.; Nenna, G.; Borriello, A.; Miscioscia, R.; Minarini, C.; Roca, F. Evaluation of the PMMA microlens efficiency for the realization of a solar micro-concentrator array. Appl. Opt. 2018, 57, 4396.
- Fujimoto, K.; Tadokoro, H.; Ueda, Y.; Ikada, Y. Polyurethane surface modification by graft polymerization of acrylamide for reduced protein adsorption and platelet adhesion. Biomaterials 1993, 14, 442–448.
- Torstensson, M.; Ranby, B.; Hult, A. Monomeric Surfactants for Surface Modification of Polymers. Macromolecules 1990, 23, 126–132.
- Wilson, C.A.; McCormick, J.A.; Cavanagh, A.S.; Goldstein, D.N.; Weimer, A.W.; George, S.M. Tungsten atomic layer deposition on polymers. Thin Solid Films 2008, 516, 6175–6185.
- Minton, T.K.; Wu, B.; Zhang, J.; Lindholm, N.F.; Abdulagatov, A.I.; O’Patchen, J.; George, S.M.; Groner, M.D. Protecting polymers in space with atomic layer deposition coatings. ACS Appl. Mater. Interfaces 2010, 2, 2515–2520.
- Kemell, M.; Färm, E.; Ritala, M.; Leskelä, M.; Färm, E.; Ritala, M.; Leskelä, M. Surface modification of thermoplastics by atomic layer deposition of Al2O3 and TiO2 thin films. Eur. Polym. J. 2008, 44, 3564–3570.
- Napari, M.; Malm, J.; Lehto, R.; Julin, J.; Arstila, K.; Sajavaara, T.; Lahtinen, M. Nucleation and growth of ZnO on PMMA by low-temperature atomic layer deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2015, 33, 01A128.
This entry is offline, you can click here to edit this entry!
