2.2.1. Selenium Intrinsic Properties Decisive in Nanophase Selection
Nanomaterials such as NPs with their small size and higher specific surface area are appropriate to be immobilized into solid matrices and to construct nanocomposites by modification of NPs with polymer chains. The embedment of NPs into polymeric matrices minimizes their mobility and their interaction with the environment. Thus, the use of nanocomposites in an effective way by increasing their stability, along with the safety of nanoparticles, is of paramount concern. An important advantage of nanocomposites is the accessibility of substrates to the functional NPs [
169].
Various inorganic fillers are widely employed to produce polymer composites exhibiting unique physicochemical properties that guarantee active effects, such as antimicrobial effects (e.g., Ag and Cu), scavenging of gas molecules (e.g., Fe or Pd), and antioxidant effects (e.g., selenium) [
32], along with the enhanced polymer’s intrinsic properties (thermal, mechanical, optical, rheological properties, etc.) [
170]. Thus, along with the metals-containing formulations, the nanoscale fillers in the form of selenium NPs were examined. Moreover, SeNPs constitute in many cases a cheaper and safer alternative because of the selenium role as an essential micronutrient required by living organisms. Selenium has found its path into the area of nanotechnology due to its remarkable biological properties, such as antibacterial, antiviral, and antioxidant characteristics [
171].
Selenium biochemistry-related studies are forced to rely on controversial mechanisms built into selenium substances’ chemical nature and intrinsic properties. That gives reasons for the contradictions found among the Se necessary as an essential microelement in human and animal organisms and its toxicity even at moderate levels. An excess amount of selenium causes toxicity and harmful consequences in humans [
172]. That is especially true and became commonly accepted about a decade ago with traditional selenium compounds, inorganic salts such as selenite and selenate, which exhibit poor application potential due to the low bioactivity and inadequate cytotoxicity to normal cells [
173]. Nanotechnology has revolutionized the field of Se-substances application by expounding SeNPs as a distinctive chemopreventive agent, which shows good selective cellular uptake and enhanced anticancer activities. The acute toxicity of SeNPs in mice is remarkably lower compared to inorganic salts or amino acids, such as selenite, selenomethionine, and selenomethylselenocysteine [
174,
175]. Along with an anticancer therapy [
176,
177,
178,
179], the major biomedical applications of SeNPs include a targeted drug delivery, drug delivery vehicles and artificial enzymes [
180,
181], and biosensors and intracellular analysis [
182]. SeNPs are known for inhibiting cellular entry of viruses while maintaining low toxicity. Several functionalized SeNPs are capable of blocking the pathogen attachment to cell and viral entry, because viral infections start with the binding of viral particles to receptors on the host cells, followed by the entry of the virus into the cells [
183]. Selenium-containing NPs, such as zanamivir-functionalized SeNPs [
184], SeNPs loaded with oseltamivir [
185], amantadine-functionalized SeNPs [
186], and SeNPs loaded with ribavirin [
187] have been examined for inhibitory effects upon and biocompatibility with the H1N1 influenza virus. The antiviral activity of SeNPs can be further enhanced when being combined with the antiviral drug arbidol [
188], effectively blocking cell entry of influenza virus and reducing cell apoptosis. Some drug-conjugated SeNPs were tested against Covid-19 infection and apoptosis inhibition properties [
183].
SeNPs can be prepared via physical methodologies such as laser ablation, UV radiation, hydrothermal techniques, etc. [
189]. Additionally, SeNPs can be synthesized via chemical methodologies such as acid decomposition, the precipitation method, reduction using ascorbic acid, sodium dodecyl sulfate, sulfur(IV) oxide and glucose, etc. Yet, despite the design of nanoparticles with a definite shape and size via these methods, they require the use of chemicals, harsh conditions like acidic pH, and high temperature, which make them unsuitable for applications in the medical field [
2]. As a type of nanomaterial with improved biocompatibility, SeNPs were reported to be obtained via the selenite anions reduction to elemental selenium Se
0 with polymeric or non-polymeric reducing agents, as chitosan– or another polysaccharide–protein complexes,
Spirulina polysaccharides,
Undaria pinnatifida polysaccharide, doxorubicin, or adenosine triphosphate [
190,
191,
192,
193,
194,
195,
196]. In the presence of different chemically obtained or pseudonatural polymers serving as a stabilizing agent, SeNPs can be synthesized via the reduction of sodium selenite with, e.g., ascorbic acid [
9] by means of a solution-phase approach. Characterization of size and morphology of the resultant NPs by TEM showed that SeNPs obtained using different stabilizers such as chitosan (poly(D-glucosamine), Triton X-100 (t-octylphenoxypolyethoxy ethanol), and ethoxylate-type additive (2,4,7,9-tetramethyl-5-decyne-4,7-diol ethoxylate) were spherical with a diameter within the intervals 20–40, 18–40, and 28–60 nm, respectively. With a stabilizer isotridecanol ethoxylate, SeNPs were of nanorods morphology and exhibited poor antioxidant properties [
9]. Subsequently, the solutions containing the selected spherical, 50–60 nm in diameter SeNPs were incorporated in a flexible multilayer plastic material to be used as food packaging. The antioxidant performance and final laminate structure stability assays showed that the Se-layer was a non-migrating, efficient, free-radical scavenger [
9].
Therapeutic benefits of SeNPs include anti-inflammatory, anti-diabetic, antioxidant, and antimicrobial action [
197]. Many studies indicate potent antitumor activity of SeNPs, their conjugates, and functionalized products [
198,
199] by inducing caspases and mitochondria-mediated apoptosis [
157]. Various conjugates of SeNPs contribute greatly to an emerging interdisciplinary area, cancer nanotechnology. Green synthesis of SeNPs with the experimentally confirmed ability to fight against a wide array of cancer types was performed with degreased walnut meal [
200], non-pathogenic bacteria [
201,
202,
203], algae [
193], yeast fermented broth [
204], and culture extract of fungus
Monascus purpureus [
205]. Mushroom’s
Polyporus rhinocerus water-soluble polysaccharide–protein complexes [
194] and hyper-branched β-
d-glucan from
Pleurotus tuber-regium [
157,
163] are examples of higher-fungal polymers as the capping agents potentiating in vivo anticancer efficacy of SeNPs. An essential advantage of biogenic SeNPs is to represent more cytotoxicity on cancer cells compared to normal cells. Very recent studies also regard evidence for the anticancer potential of biogenic SeNPs and discuss the proposed anticancer mechanisms of this nanomaterial [
206].
The known surface chemistry of the mycogenic SeNPs would lead to the possibility of selecting the reducing molecules and capping agents and thereby control the size and shape of the NPs. In this respect, the chemical form of Se
0 precursor (nanophase producing) compounds should be considered as a limiting factor determining the subsequent surface modification of neonatal SeNPs in solution. SeNPs show attractive reduced toxicity compared with selenium bulk counterpart (bulk selenium) [
207]. Nevertheless, the native SeNPs suffer from the issue of stability, which leads to compromised efficiency and physicochemical characteristics of these nanostructures [
208].
2.2.2. Mechanisms Underlying the Occurrence of Selenium Nanophase
Various attempts have been made to improve the stability and biocompatibility of SeNPs. The latter form aggregates, which may have led to less availability and efficacy, resulting in the requirement for a high concentration [
209]. To obtain an excellent integration between organic and inorganic phases in nanocomposites, tuning specific procedures should be implemented that allow one to achieve strong polymer–filler interaction. Much effort has been exerted to develop a “green” method of synthesis and dispersion of SeNPs. The generally accepted approach is that the key mechanism behind a green synthesis of NPs is a microorganism- or plant-assisted reduction of precursor compound owing to various metabolites [
210], with not one biomolecule but several secondary metabolites together being accountable [
211]. Ligand capping agents, otherwise called stabilizing or functionalizing agents, are molecules that bind to the nanoparticle surface to confer stability and prevent nanoparticle aggregation [
212]. Unlike physicochemical methods of nanoparticle synthesis, which require additional steps for surface functionalization, in biogenic methods, synthesis and capping occur simultaneously [
213]. Likewise, in fungi, biopolymers play a vital role as reducing and capping agents [
214]. However, there are few reports on the exact characterization and identification of the capping biomolecules in biogenic NPs. Fungal proteins, enzymes, cofactors, and other metabolites play crucial roles in the organism’s survival and reduce precursor compounds to cause a corresponding chemical element, including selenium, zero-valent nanoparticulate forms.
NPs are assembled from the corresponding atoms, molecules, and clusters using chemical or biological techniques, these constructive procedures being called the bottom-up method in contrast to the top-down (destructive) method of the NPs’ preparation [
215]. The bottom-up approach provides definite advantages in terms of better control over the final product formation and homogeneity of physicochemical parameters. Disadvantages inherent to this approach, namely the necessity of separation and purification of the synthesized particles from their reaction mixture, toxic chemicals, organic solvents, and reagents [
216], is a serious challenge. The exception is the green synthesis method, in which bottom-up protocol-based procedures of NPs synthesis may be performed under ecofriendly conditions.
NPs can be generated by microbes either intracellularly or extracellularly. Several reviews focus on the bioproduction of metal NPs by fungi [
25] and other microorganisms, both unicellular and multicellular [
217], to explore the chemistry of inorganic nanomaterial formation through these two methods. In both cases, during the SeNP synthesis, selenium is involved in the nutrient exchange and/or substance diffusion. The microbial cells prevent damage by producing specific metabolites and electrons, which can reduce the Se-containing precursor compound to yield selenium in a higher (zero) oxidation state. The nuclei grow and thereby form nanoparticles intracellularly or extracellularly. Using fungal cells, biosynthesis of SeNPs follows either of the two mechanisms.
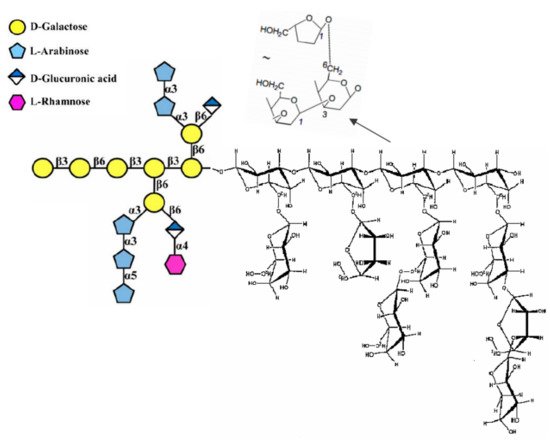
In intracellular synthesis, NPs are formed and localized in the cytoplasm, cell wall, or cell membrane. [
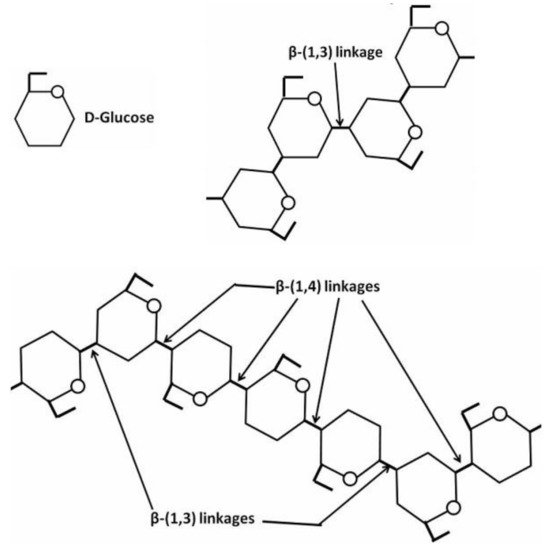
24]. The fungal cell wall typically contains glucans, glycoproteins, and chitin, which are bonded by inter- and intra-molecular hydrogen-bonding between these units. Glucans contain the predominantly linear β-1,3-linkage and a small portion of β-1,6- and β-1,4-linkages. Chitin and α-1,3-glucan build a hydrophobic scaffold that is surrounded by a hydrated matrix of diversely linked β-glucans. Glycoproteins and a minor fraction of α-1,3-glucans form a highly dynamic shell coating the cell wall surface [
218]. Hence, owing to its composition, the fungal cell wall along with various proteins plays a central role in the bioreduction responsible for NPs’ appearance. Alternatively, the NPs or precursor ions may diffuse through the cell membrane and be reduced by redox mediators in the cytoplasmic matrix [
219]. Intracellular production of metal NPs by fungi was known for a relatively prolonged period. At the very beginning of the 21st century, the intracellular preparation of AuNPs using a fungus (
Verticillium sp.) was first reported by Mukherjee et al. [
220], where Au
3+ ions from tetrachloroaurate were reduced within the fungal cells, resulting in the formation of particles within the size range of 20 nm. In the process of intracellular mycogenic synthesis of SeNPs, selenium as a precursor material of NPs is reduced by the fungal biomolecules present in the cell wall to yield NPs, which are formed on the surface of mycelia, not in the nutrient medium. This method presumes that a Se-supplying compound is delivered inside the targeted fungus and creates SeNPs in the company of fungal reducing agents. This nanoparticle precursor (Se-containing compound) first interacts with oppositely charged cell surface moieties, where it could be simultaneously reduced to respective SeNPs and remain bound to the cell surface [
32]. Such NPs may diffuse to the cell membrane or cytoplasm. Alternatively, a Se-supplying compound could be internalized by active or passive transport inside the cell, and selenium in zero oxidation state occurs on account of intracellular reducing agents.
Since the intracellularly produced NPs are synthesized by microorganisms including fungi, the purity of particles from this process can be questionable. There is a high possibility that the NPs obtained are associated with the microorganism itself, various microbial cellular components, or both [
221,
222,
223]. Multicomponent residuals from microorganisms accumulated on the NPs surface would also trigger potential immunological reactions when exposed to living systems [
16]. Intracellular processes suffer from a significant disadvantage in terms of product recovery that makes the process hard and expensive, since NPs bind to the cell, and certain treatments such as cell disruption or solvent extraction are required to isolate the generated NPs [
48]. As SeNPs can also be formed intracellularly, the separation of these particles from the fungal biomass without altering their properties is extremely challenging. The ideal situation would be that SeNPs are produced extracellularly, which turns out to be more environmentally friendly and cost-effective [
224].
The extracellular synthesis of NPs was first reported by Shahverdi and co-workers [
225], where AgNPs were produced by the reduction of aqueous Ag
+ ions through various culture supernatants of Gram-negative bacteria. Extracellular mycogenic synthesis of SeNPs involves the bioreduction of the selenium-supplying compound to elemental selenium, which may be stabilized by organic molecules presented in fungal culture liquid, and SeNPs are formed outside the fungal cells. These processes were long ago known to be performed by different biopolymers [
226] and other active components including amino acids, vitamins, alkaloids, polyphenols, flavonoids, and organic acids [
227], which can act both as reducing and capping agents during the NPs formation, thus promoting the synthesis of NPs and inhibiting their agglomeration [
228]. Zhang et al. [
229] have demonstrated that the presence of a protein such as bovine serum albumin in the redox system can control the aggregation of elemental selenium atoms, thus inhibiting the formation of bulky red elemental selenium particles and consequently leading to the formation of red elemental selenium nanoparticles. However, the exact events of SeNPs synthesis have not been elucidated yet.
The cellular defense mechanisms manifested by the fungus-mediated NPs’ formation were noted some considerable time ago, mainly during the metal NPs’ mycosynthesis studies [
34,
35]. It is supposed that fungi will take measures when the toxic ions are present in their growth environment to provide protection [
230]. In doing so, fungi secrete extracellular metabolites to be used to remove unwanted Se-containing substance in the form of SeNPs. The exhibited defense mechanisms can be categorized again into intracellular and extracellular means. The former facilitates the reduction of the negative effects of entrapped Se-supplying substance by binding with biomolecules or efflux channels, while the latter implies the inhibition of the uptake and internalization of foreign Se-containing matter [
24].
Fungal synthesis of NPs takes place with nitrate-dependent reductases and electron shuttle quinones [
231]. Fungi produce napthoquinones and anthraquinones [
232,
233,
234,
235], which act as reducing agents. Many filamentous fungi elaborate a large number of simple hydroxyl or methoxy derivatives of benzoquinones, toluquinones, or quinoline [
219] as a response to abiotic stress. For reduction processes, not only the biocatalyst, enzyme, is necessary, but also an electron shuttle. Membrane-bound oxidoreductases play a crucial role in the process along with different quinones [
236]. It was speculated that a conjugation between the quinone electron shuttle with the reductase somehow facilitated the formation of NPs [
237]. As early as 2010, Jha and Prasad [
236] found that the presence of these metabolites may generate a redox reaction due to tautomerization, leading to the production of nanomaterials. The aforementioned processes transfer electrons to the quinone pool. Additionally, it was expected that fungi secrete a reduced nicotinamide adenine dinucleotide (NADH) as one of the components of the reducing agents moiety [
26], which along with other ingredients, reduce the precursor substances to the corresponding NPs. In order to confirm their hypothesis, NADH alone and NADH along with a fungal extract were added to the solution with the precursor agent (to be reduced to yield NPs). The researchers did not observe any change in color for NADH alone. However, when NADH accompanied by a fungal extract was added, the reaction started after a few minutes [
26]. The assays show that NADH could be a key factor in the synthesis of NPs, but other molecules are essential, perhaps, as a biocatalyst of the redox reaction [
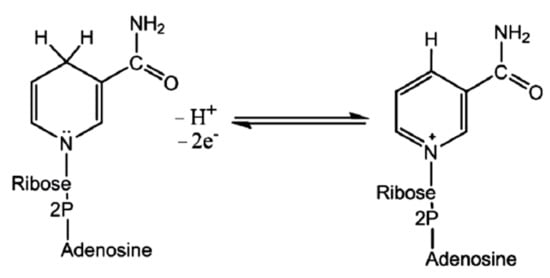
238]. The reductase enzyme obtains its electrons from NADH oxidation to an oxidized nicotinamide adenine dinucleotide (NAD
+) (Figure 4).
Figure 4. Reversible transitions among NADH and NAD+.
During the oxidation, the enzyme also becomes oxidized simultaneously, resulting in NPs’ synthesis. Since NADH acts as an electron carrier and selenium in Se-supplying precursor as an electron acceptor, the reduction of positively charged Se species to SeNPs occurs. It has been observed that the nitrate-dependent reductase can also participate in the bioreduction [
215]. Some studies reported that NADH-dependent reductases could be specific to a fungal species [
42,
239], e.g., reductase specific to
Fusarium oxysporum enzyme was believed to catalyze the facilitated NPs formation [
240,
241]. Thus, the above-mentioned extracellular defense mechanism behind the SeNPs mycosynthesis is believed to involve NADH-dependent enzymes, notably nitrate reductases, which are secreted into the reaction medium together with electron shuttles such as quinones.
Smaller-molecular-mass substances such as ascorbate, citrate [
242], cofactors, glucose, and amino acids [
243] could also serve as reducing agents, which stabilize the resulting NPs in fungal biosystems. Some studies dealing with the fungi-assisted fabrication of SeNPs suggest electrostatic interaction of free amine groups or cysteine residues with NPs’ surface [
244,
245,
246,
247]. The electrostatic interaction followed by secretion of such metabolites as extracellular polymeric substances that can adhere to unwanted foreign matters (NP material) [
248] are the first steps of the fungal SeNPs’ formation mechanism. Stimulation of fungal development by Se-containing molecules is owing to the fungus capability of binding and stabilizing key compounds for mycelium growth, whose molecules have various chemical structures, dimensional and charge characteristics, solubility, lipophilic properties, and reactivity. The distribution of charges plays a certain role. The negative charge on the mycelium surface, which promotes the complexation process during selenium–ligand interactions, is provided by chitin, a component of the fungal cell wall [
249], and carboxyl, amine, thiol, amide, imine, thioether, and phosphate functional groups [
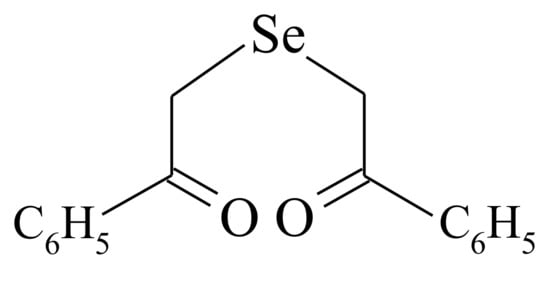
250]. That is why the electrostatic interaction is believed to happen between the positively charged Se-sourcing matter (inorganic Se-salts or organic selenide DAPS-25) and the negatively charged groups on the fungal cell. While the electrophilic character of Se(IV) and Se(VI) is of no doubt, the electrophilicity of Se(II) in the molecule of selenide (Figure 3) is confirmed by the positive value of the natural charge on the selenium atom, as was computed by Pankratov A.N. [
247].
Molecular mechanism studies suggested that certain NPs inactivate proteins by binding with -SH (thiol) groups of proteins in the cell. Tripeptide L-γ-glutamyl-γ-cysteinyl-glycine, glutathione (GSH), contains an active thiol group of a cysteine residue. GSH secretion is a very important element of redox homeostasis [
251]. Due to a sufficient negative value of redox potential, GSH acts as a buffering agent in the eukaryotic cells [
19]. It has been found that GSH secretion under abiotic stress initiates the intracellular detoxification pathway. Several schemes are known for the reaction of organoselenium compounds with thiols. In the work [
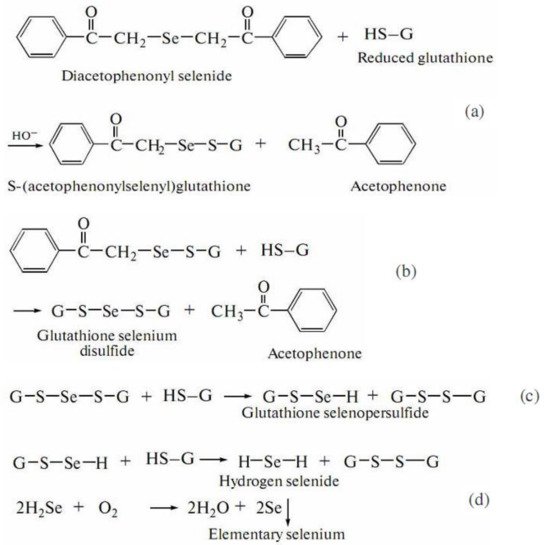
246], aimed at the investigation of a reaction between DAPS-25 (Figure 5) and cysteine or reduced glutathione for their identification by the ascending thin-layer chromatography technique, a scheme for the interaction of diacetophenonyl selenide with the compound-bearing sulfhydryl group was proposed (Figure 6).
Figure 5. Structure of the compound DAPS-25.
Figure 6. Scheme of DAPS-25 interaction with thiols.
The half-products, acetophenone and S-(acetophenylselenyl)glutathione, form in the first step (Figure 6a). The next step is the formation of one acetophenone molecule and glutathione selenodisulfide (Figure 6b). Then, glutathione selenopersulfide and glutathione disulfide form from selenodisulfide in an excess of reduced glutathione (Figure 6c). The final step is the formation of hydrogen selenide. The resulting hydrogen selenide as a strong reductant can be oxidized to elementary selenium by both air oxygen and glutathione disulfide (Figure 6d) [
246]. Therefore, for an organoselenium compound to be decomposed rapidly under the action of reduced glutathione or cysteine to release elementary selenium, its molecule should contain the –CO–CH
2–Se–CH
2–CO– group.
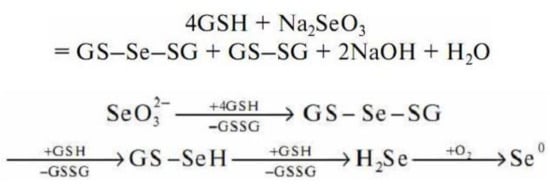
Sodium selenite undergoes a non-enzymatic reaction with GSH to form selenodiglutathione (GS–Se–SG) [
252] (Figure 7).
Figure 7. Scheme of sodium selenite interaction with reduced glutathione.
According to the general scheme of the redox reaction, in an excess of GSH, selenodiglutathione is readily reduced to form selenopersulfide (GSSeH) and then hydrogen selenide. Hydrogen selenide oxidizes to elemental selenium.
Thus, there are fungal protective mechanisms to resist the toxicity of the Se-precursor compounds by their immobilization in a less-toxic Se0 nanoparticulate form. The eukaryotic microbial system as fungus has enough strategies to survive with selenite stress. Exposure to the Se-sourcing agent solution prompts the fungus to produce metabolites to overcome xenobiotic-induced stress. In this process, the Se-supplying substances are reduced to SeNPs through the catalytic effect of the extracellular enzymes and by implementing the fungal metabolites. The defense option applied by fungi to survive in the presence of unwanted Se-agent consists in the binding of Se-ions to high-affinity functional groups, with thiols, such as cysteine residues and GSH, contributing greatly to this fungal strategy. An elemental selenium accumulation presents the final stage (Figure 6 and Figure 7).
Various organic and inorganic chemical compounds may serve as a reducing agent implemented in mechanisms of SeNPs mycosynthesis. Along with the low-molecular-weight compounds considered above, mushroom biopolymers, mainly bioactive polysaccharides, could serve as the capping agent during the reduction of selenium salts [
194] and organoselenium substances. Fungal polysaccharides could engage the surface of SeNPs to prevent particulate aggregation and control their particle size [
157]. Strong physical adsorption of hydroxyl groups on selenium surfaces aids in the development and stabilization to construct water-dispersible SeNPs capped with a polymeric agent in an aqueous system [
163]. However, the precise processes are unclear, and further research should provide a deeper understanding of the molecular mechanisms involved in the occurrence of selenium nanophase.
2.2.3. Points for Physicochemical Characterization of Culture Conditions and Resultant SeNPs
Before being implemented in any branch of technology, the nanomaterial needs to be fully understood and characterized [
253]. The physicochemical characterization of NPs complements their biochemical characterization. Many articles describe techniques that are effective in the characterization of nanostructures and nanomaterials. Particle attributes such as composition, size distribution, morphology, surface chemistry, homogeneity, stability, etc., are crucial for determining the biotechnological potentialities and the impact of the NP-comprising matter on the environment. Reliable analytical tools employed for SeNP characterization yield multidimensional information on these nanostructures. The anthropogenic and naturally occurring SeNPs are usually characterized via several techniques such as ultraviolet-visible (UV-Vis) absorbance spectroscopy, light-scattering-based techniques (dynamic light scattering (DLS) and nanoparticles tracking analysis (NTA)) [
153], Fourier transform infrared (FTIR) spectroscopy [
254], transmission electron microscopy (TEM) and field emission scanning electron microscopy (FESEM) [
9], X-ray diffraction (XRD) measurements, and Raman spectroscopic techniques [
255]. Qualitative analysis for selenium is frequently carried out with electron energy loss spectroscopy in the TEM (EELS-TEM) accompanied by a chromatographic (gas chromatography coupled with mass spectrometry, GC-MS) detection of the Se-supplying compound (bio)transformation in the course of the biosynthetic process [
81]. Hyphenated techniques are efficiently applied for selenium speciation, e.g., inductively coupled plasma mass spectrometry (ICP-MS) [
256] in single-particle mode (SP-ICP-MS) or coupled with asymmetric flow field-flow fractionation (AF
4) in AF
4-ICP-MS [
257], and continuous photochemical vapor generation (PCVG) coupled with microwave-induced plasma optical emission spectrometry (MIP-OES). Bartosiak et al. applied the yeasts
Saccharomyces boulardii to SeNPs bioproduction and performed calculation of accurate NP yield [
258] A selective identification and quantitative determination of both the unreacted precursor Se(IV) and the resultant NPs without the need to separate them were enabled by means of the PCVG- MIP-OES technique.
External physicochemical parameters of the Se
0 producing fermentation, such as acidity, temperature, aeration, Se-supplying compound type and concentration, etc., are found to be important factors that could be decisive for the SeNPs nanoscale features. Well-dispersed spherical 60 nm sized SeNPs synthesized at pH 8 had an average diameter value of 300 nm in strongly acidic conditions [
259]. Acidity parameters close to those of neutral solutions (pH 7 to 8) [
260] or a somewhat wider pH range (pH 6 to 9 for precursor selenite and pH 7 to 9 for selenate salt) [
261] were reported to facilitate the SeNPs synthesis. However, for some microorganisms used in green techniques, far-from-neutral media can be appropriate. The corresponding pH range was as broad as 4 to 10 when bacterium
Acinetobacter sp. SW30 was implemented for SeNPs bioproduction [
201]. In this research, the SeNP formation process occurred under thermal conditions up to 40 °C, and much higher temperature (80 to 100 °C) appeared to cause the SeNPs’ aggregation into nanorods. The effect of broad acidity parameter (pH 5 to 12) on the expression of proteins in the fungus
Mariannaea sp. HJ in the presence of selenium oxide SeO
2 as a Se-precursor was demonstrated [
262]. It was indicated that the higher the acidity, the greater the total protein concentration, and both maxima were reached at pH 10–11 and 198 mg/L, respectively. Further increase in pH value led to a drastic decrease in the total protein concentration. Thus, pH could affect the expression of fungal proteins capable of alleviating alkaline condition stress in the fungus. The Se-sourcing agent’s concentration along with the cultivation pattern of biosynthesizing microorganism manages the size, shape, and cellular location of SeNP [
255]. Spherical and rod-like SeNP shapes were produced at 3.0 mM of sodium selenite in the medium, whereas Se-spheres were solely found at 1.5 mM of the same starting Se-compound [
201]. Aeration is one of the influencing factors here. Diko et al. reported the synthesis of spherical SeNPs (≤200 nm in diameter) using
Pseudomonas stutzeri, which reduced sodium selenate more rapidly under anaerobic conditions, while Se(IV) salt was not reduced at all. Strain NT-I was able to tolerate elevated concentrations of inorganic Se-salts. Bacterium reduced selenate completely at its level of up to 10 mM and selenite almost completely (up to 9 mM salt concentration). In addition, even higher concentrations of these Se-salts were substantially reduced by the bacterium [
263]. Moderate thermal (20 to 50 °C) and acidity (pH 7–9 or 6–9 for selenite and selenate, respectively) conditions were favorable for bioreduction to yield elemental selenium.
Since common physicochemical factors control the cellular location of SeNPs, both the intracellular and extracellular locations of mycosynthesized SeNPs were observed by many researchers [
33,
262,
264]. Thus, filamentous fungi
Aureobasidium pullulans,
Mortierella humilis,
Trichoderma harzianum, and
Phoma glomerata were capable of intra- and extracellular bioproduction of SeNPs [
33]. The red deposit was confirmed as elemental selenium during fungal growth on Se-containing media. Along with Se
0 formation, selenium oxide was found in the case of
Trichoderma harzianum culture with 1 mM selenite. Both particle sizes and SeNPs concentrations were determined by the SP-ICP-MS technique. Only
A. pullulans and
M. humilis produced SeNPs in 10-day culture, with diameters ca. 60 and 48 nm, respectively. In 20- and 30-day cultures, particles were enlarged in diameter to reach about 78 and 61 nm. Using the SP-ICP-MS method, only a low SeNP level was detected in
T. harzianum and
P. glomerata supernatants of liquid cultures [
33]. Rosenfeld et al. showed that the ascomycete fungi (
Pyrenochaeta sp.,
Acremonium strictum,
Plectosphaerella cucumerina,
Stagonospora sp.,
Alternaria alternata,
Paraconiothyrium sporulosum) produced Se
0 by Se inorganic salts reduction, with the NPs being strongly bound by the fungal biomass for all six species used [
264]. SeNPs’ diameter ranged from 50–100 nm (
Alternaria alternata) to 200–300 nm (other fungal species). Particles were spherical. Results of XRD analysis demonstrated that the diffraction patterns of all SeNPs were X-ray amorphous, although two of the analyzed extracellular SeNPs mycosynthesized by
Acremonium strictum were crystalline with a d-spacing of 0.37 nm. Residual dissolved Se(IV) and Se(VI) concentration values were determined by conductivity measurements using an ion chromatography system enabling a simultaneous quantification of both oxyanions, comprising Se(IV) and Se(VI), with a detection limit of micromole/L for each anion. Analysis for total selenium associated with fungal biomass was performed using the inductively coupled plasma optical emission spectroscopy (ICP-OES). The presence of Se
0 was confirmed by SEM EDS. Transmission electron microscopy revealed intracellular and extracellular SeNPs in all fungal species used [
264]. Biosynthesis of SeNPs by
Mariannaea sp. HJ was reported for the first time very recently [
262]. Various culture conditions were studied, including SeO
2 concentrations and pH of reaction media. To characterize the resultant SeNPs, the multimethod approach was used: UV-Vis, TEM, SEM, XRD, FTIR, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were used. TEM images depicted the SeNPs’ deposition both on the fungal cell wall and in the cytoplasmic region, thus suggesting the biotransformation of Se(IV) into elemental selenium to occur in extracellular and intacellular locations, which could provide a template for these bioreduction processes [
262].