Intercellular communication governs multicellular interactions in complex organisms. A variety of mechanisms exist through which cells can communicate, e.g., cell-cell contact, the release of paracrine/autocrine soluble molecules, or the transfer of extracellular vesicles (EVs). EVs are membrane-surrounded structures released by almost all cell types, acting both nearby and distant from their tissue/organ of origin. In the kidney, EVs are potent intercellular messengers released by all urinary system cells and are involved in cell crosstalk, contributing to physiology and pathogenesis. Moreover, urine is a reservoir of EVs coming from the circulation after crossing the glomerular filtration barrier—or originating in the kidney. Thus, urine represents an alternative source for biomarkers in kidney-related diseases, potentially replacing standard diagnostic techniques, including kidney biopsy.
1. Introduction
The kidneys are the primary filtrating system in the human body. They finely regulate the extracellular fluid volume by altering water excretion and electrolyte concentration, controlling the elimination of waste products, and showing multiple endocrine functions associated with the direct or indirect synthesis of hormones [
1].
Kidney diseases, in most cases, are related to multifactorial pathologies such as diabetes and hypertension, as well as immune-mediated conditions. Tests that measure kidney function permit recognition of the damage, monitoring its progression and response to treatment [
2]. Kidney biopsy represents a specific diagnostic tool, but it is an invasive and hardily iterative technique, especially in specific patient subgroups, such as children and older people. Recently, urinary peptides [
3] and microRNAs (miRNAs) [
4] have been suggested as valid alternative markers to classical biochemical measurements [
5] of kidney disease progression. Both proteins and miRNAs have been described to be relatively stable in biological fluids, mainly when carried by extracellular vesicles (EVs).
EVs are membrane-surrounded structures released by all cell types and are found in body fluids [
6]. EV composition incorporates various bioactive molecules—including membrane receptors, soluble proteins, nucleic acids, and lipids—which can be transferred to target cells [
7]. EVs are involved in cell-to-cell communication, influencing recipient cells via direct (i) receptor-binding, thereby transducing a signal, (ii) endocytosis or (iii) fusion with the cell membrane to transfer their molecular contents. EVs may also modify target cells’ microenvironments (i) by interacting with the extracellular matrix (ECM) through integrins or CD44 and (ii) by releasing growth factors/chemokines, thanks to the presence of active enzymes on their surface [
8]. EVs have been implicated in cell growth and differentiation, angiogenesis and coagulation, immune modulation, and inflammation [
9].
Some of the fundamental qualities of a promising biomarker include good stability, easy accessibility, and the absence of invasiveness during its collection [
10]. Based on this approach, EVs could represent an innovative tool for the so-called liquid biopsy, a technique allowing clinicians to screen nonsolid biological tissues such as blood or urine, searching for markers to assess a patient’s condition [
11]. Urine contains a large amount of EVs, including those that cross the glomerular filtration barrier (GFB) from circulation, ending up in the urine, and also those originating from the kidney itself. Thus, urine presents itself as an optimal source for biomarker discovery in kidney diseases [
12].
2. Extracellular Vesicles
The term extracellular vesicles (EVs) categorizes vesicles based on their size (exosomes, microvesicles, and apoptotic bodies) and release pathways [
13]. This review will refer mainly to exosomes and microvesicles, which are the most studied EV populations (Table 1).
Table 1. Overview of EVs: their size, origin, composition and isolation techniques.
| |
Exosomes |
Microvesicles |
| Size (nm) |
20–150 |
100–1000 |
| Origin |
MVB fusion with plasma membrane |
Outward blebbing of plasma membrane |
| Release process |
Constitutive/Cellular Activation |
Constitutive/Cellular Activation |
| Pathways |
Tetraspanins-dependent |
Cytoskeleton reorganization- and Ca2+-dependent |
| Composition |
|
|
| Proteins |
CD9, CD63, CD81, Alix, TSG101, Rabs,
annexins, MHC-II, CD86, signaling-,
oncogenic-, integrin-, adhesion
molecules, flotillins, Hsp70 and Hsp90 |
Annexins, flotillins, Alix, TSG101,
CD40, ARF-6, selectins,
phosphatidylserine, Rho family
members, MMP2, ADAM, GAPDH,
pyruvate kinase, proteins localized to
centrosome, nucleolus, cytoplasm and
mitochondria |
| Nucleic Acids |
mRNAs, miRNAs, lncRNAs, mtDNA,
gDNA, nDNA, dsDNA |
mRNAs, miRNAs, gDNA |
| Isolation Method |
UC, DG-UC, filtration, immune-
affinity, PEG precipitation, SEC |
UC, DG-UC, filtration, SEC |
Exosomes are small membranous vesicles of endocytic origin ~20–150 nm diameter. In comparison, microvesicles (100–1000 nm in diameter) are large membranous vesicles that are shed directly from the cell plasma membrane and are more heterogeneous in shape and dimension [
14].
The release of EVs can occur physiologically or can be elicited by paracrine factors (cytokines and chemokines) [
15,
16,
17], or physical, chemical, or mechanical stress-stimulated cells [
18,
19]. EV secretion can also vary in response to external stimuli such as hypoxia [
20], inflammation [
21,
22], and pH variation [
23]. Since EVs in individuals with different pathological states are higher than in healthy subjects and show unique signatures [
24,
25], they have been described as correlating with worsening of the disorders.
Exosomes can generate from intra-luminal vesicles (ILVs) inside multivesicular bodies (MVBs), whereas microvesicles are mainly produced via outward blebbing of the plasma membrane through an asymmetric distribution of cell membrane phospholipids. The complex pathways involved in exosome and microvesicle biogenesis are outside of the scope of this review and have been summarized in the following [
26,
27,
28].
3. EVs in the Kidney: Mechanism of Cell-to-Cell Communication
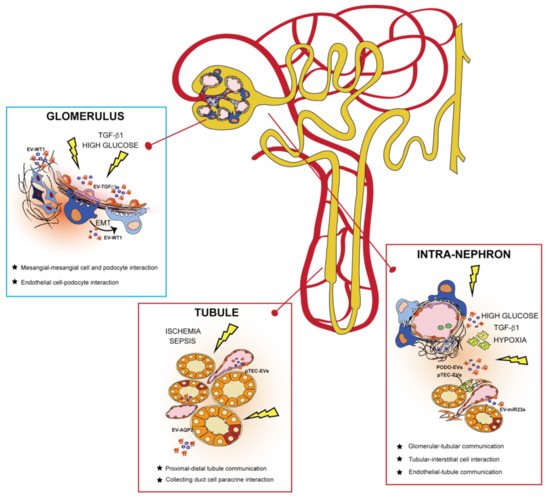
EVs are potent intercellular messengers carrying different molecular cargos implicated in communicating signals throughout the nephron (Figure 2) [
70]. In this context, all the cells composing the urinary system can release EVs involved in cell crosstalk, contributing to kidney physiology and pathogenesis [
71].
Figure 2. Extracellular vesicles (EVs) govern intracellular communication between intra-nephron compartments. EVs released from different glomerular cells and tubule segments can interact in the same physical compartment or mediate communication from distant nephron sections in physiological and pathological conditions. In the glomerulus, EVs released from mesangial and endothelial cells (such as EV-TGFβ) mediate a paracrine response to injury by affecting neighboring kidney cells. EVs of podocyte origin (EV-WT1) are released in the urine during pathological conditions, behaving as biomarkers of glomerular kidney damage. In the tubule, EVs are secreted by tubular epithelial cells (TECs) to maintain fluid homeostasis and electrolyte balance (EV-AQP2) in the body. After an injury, EVs from both podocytes (PODO-EVs) and proximal TECs (pTEC-EVs) mediate intra-nephron interaction with interstitial fibroblasts and tissue-infiltrating macrophages, promoting progression to chronic kidney disease.
4. Urinary EVs as Biomarkers for Personalized Medicine
In clinical practice, the most commonly used markers of kidney disease, including serum creatinine, estimated glomerular filtration rate (eGFR), blood urea and proteinuria, and albuminuria, are insufficient. The main limitations are the inability to reflect early functional changes in the kidney and the lack of prediction of chronic kidney disease occurrence [
94]. Therefore, identifying novel biomarkers, whether used alone or in combination with conventional ones, can overcome these limitations, facilitate a better definition of kidney pathophysiology and make personalized therapeutic treatments possible. In this context, urine contains a large amount of EVs, both in physiological and pathological conditions, which can reflect alterations in specific cellular compartments [
95]. Moreover, because of the glomerular architecture, large circulating EVs in physiological conditions cannot cross the GFB. Therefore, urinary EVs (uEVs) mainly originate from epithelial and parenchymal cells facing the urinary space [
22]. For this reason, they have been proposed as promising, non-invasive liquid biopsies for a variety of kidney diseases [
96]. Moreover, the optimization of protocols for the purification of exosomal RNA has allowed for their use as a novel class of easy-to-obtain sources of genetic biomarkers [
65].
5. uEVs as a Liquid Biopsy in Kidney-related Diseases
The specific composition of uEVs has been directly correlated to some primary kidney diseases. In addition, a series of diseases that secondarily affect the kidney and progress to chronic kidney disease, are demanding new non-invasive biomarkers of disease progression. The use of uEVs could represent both an early diagnostic approach and a prognostic tool capable of facilitating individual therapeutic decisions [
97]. In Table 2, we reported published papers in which uEVs were applied for the clinical diagnosis of kidney-related diseases.
Table 2. uEV biomarkers for clinical diagnosis of kidney-related diseases: a summary of human studies.
| Disease |
No. of Patient |
Target |
uEV Isolation |
Detection Method |
Main Results |
Ref. |
| |
25 patients and 5 HD |
WT1 |
Differential UC |
WB |
-WT1 was increased FSGS patients compared with healthy volunteers or SSNS patients.
-Urinary exosomal WT1 was decreased in patients in remission for either FSGS or SSNS or following steroid treatment in six SSNS subjects. |
H. Zhou, 2013
[36] |
| Nephrotic syndrome (NS) |
40 |
WT1 |
Differential UC |
WB |
-WT1 was detected in 25 patients (62.5%).
-No significant correlation between WT1 amount and steroid responsiveness or renal pathological condition was found. |
H. Lee, 2012 [37] |
| 13 |
miR-193a |
-ExoQuick exosome precipitation (System Biosciences) |
-qRT-PCR
-ROC analysis
-WB |
-miR-193a was higher in children with primary FSGS than those in children with MCNS. |
Z. Huang, 2017
[98] |
| 129 NS children and 126 age-/sex-matched HD. |
miR-194-5p, miR-146b-5p, miR-378a-3p, miR-23b-3p and miR-30a-5p |
UC |
-High-throughput Illumina sequencing
-qRT-PCR |
-These 5 miRNAs were increased in NS and markedly reduced during the clinical remission period.
-The concentrations of miR-194-5p and miR-23b-3p were positively correlated with the urine protein content and were markedly higher in the high urine protein group than in the low urine protein group. |
T. Chen, 2019
[99] |
| Glomerulonephritis (GN) |
12 IgAN, 12 TBMN patients and 6 HD |
ANPEP, VASN, A1AT and CP |
Differential UC |
Label-free LC-MS/MS |
-These four proteins are biomarkers to distinguish between early IgAN from TBMN. |
P. G. Moon, 2011
[100] |
| 55 IgAN patients and 24 HD |
CCL2 mRNA |
Differential UC |
qRT-PCR |
- CCL2 was specifically expressed in uEVs of patients with GN compared to healthy controls.
- Exosomal CCL2 was correlated with tubulointerstitial inflammation and C3 deposition in GN patients. |
Y. Feng, 2018
[101] |
Lupus nephritis
(LN) |
13 LN patients and 8 HD |
miR-26a |
Differential UC |
qRT-PCR |
-miR-26a levels in urinary exosomes were higher compared with healthy control. |
O. Ichii, 2014
[46] |
| 41 SLE patients, 27 LN and 20 HD |
miR-146a |
UC |
qRT-PCR |
-Compared to controls, urinary level of miR-146a was higher in SLE patients. |
J. Perez-Hernandez, 2020
[102] |
| 32 s patients with biopsy-proven LN, 15 non-lupus CKD and 20 HD |
miR-29-c |
Differential UC |
qRT-PCR |
-miR-29c correlated with the degree of renal chronicity but not with renal function.
-MiR-29c expression levels could predict the degree of chronicity in patients with LN. |
C. Solé, 2015
[103] |
| 31 patients |
let-7a and miR-21 |
UC |
qRT-PCR |
-Urinary exosome-associated miRNA, let-7a and miR-21, could be used to guide the clinical stage of LN patients and possibly plays a role in epigenetic regulation of the kidney during the disease. |
P. Tangtanatakul, 2019
[104] |
| Ciliopathies |
12 ciliopathy patients and 12 age- and gender-matched HD |
156 differentially expressed proteins |
Differential UC |
-Electro-phoresis
-In-depth label-free LC-MS/MS proteomics analysis |
-The most overexpressed or downregulated proteins in uEVs (VCAN, DPEP1 and FAT4) correlated with nephronophthisis-related ciliopathies. |
M.F. Stokman, 2019
[105] |
| 1 ADPKD patient and multiple HD |
PC1, PC2,
TMEM2 |
-Differential UC
- DG-UC |
Gel electro-phoresis |
In patients with PKD1 mutations, levels of PC1 and PC2 were reduced.
-TMEM2 was higher in individuals with PKD1 mutations.
-The PC1/TMEM2 ratio correlated inversely with height-adjusted total kidney volume in the discovery cohort.
-The ratio of PC1/TMEM2 or PC2/TMEM2 could be used to distinguish individuals with PKD1 mutations from controls in a confirmation cohort. |
M.C. Hogan, 2009
[106] |
| Diabetic nephropathy (DN) |
48 type-1 diabetes mellitus patients and 25 HD |
WT1 |
UC |
WB |
-WT1 expression in in uEVs was higher than in controls.
-WT1 levels were associated with an increase in urine protein-to-creatinine ratio, albumin-to-creatinine ratio, and serum creatinine as well as a decline in eGFR. |
A. Kalani, 2013
[107] |
| 30 HD and 30 T1D |
miR-424 and miR-218 |
combined centrifugation |
RT-qPCR |
Association of urinary exosomal level of miR-424 and miR-218 with renal damage in T1D patients. |
Q. Kong, 2019
[108] |
| 48 |
uEV miRNAs |
qEV size exclusion columns
(Izon Science) |
NGS |
Identification of a set of miRNAs with concentration changes associated with DN occurrence, microalbuminuria status, and other variables. |
V. Ghai, 2018
[109] |
| Obstructive nephropathy |
27 patients and 20 HD |
AQP2 TGFβ-1
L1CAM |
Centrifugation |
Immuno-blotting |
In boys with PUV, AQP2, TGFβ-1 and L1CAM correlated with eGFR. |
P. Trnka, 2012
[110] |
| 3 UPJO and 3 healthy fetusis |
633 differentially expressed proteins were identified in the amniotic fluid-derived exosomes from patients with UPJO. |
Exosome Extraction Kit |
iTRAQ quantitative proteomic profiles |
- ACE and AP-N were significantly decreased in the amniotic fluid exosomes of women with a fetus diagnosed UPJO.
- They correlate with suppressed cell proliferation, elevated ROS production, and increased pro-inflammatory cytokine levels in tubular cells. |
R. Liu,
2020
[111] |
6. EVs in Clinics: Perspectives and Limitations
In the past years, human-derived naïve and engineered exosomes have been applied in clinical trials as therapeutics for cancer and genetic diseases [
130]. In the context of biomarker discovery, the scientific community has shown a growing interest in developing standardized methods for EV studies.
For EV diagnostic applications, the established assays should be sensitive, rapid and capable of specifically detecting the biomolecules present in the vesicles [
131]. Although uEVs represent only 3% [
132] of the entire urinary proteome, a growing interest in their use as diagnostic and prognostic biomarkers has been highlighted. The translational use of EVs implies their clinical specificity. Although numerous molecular cargos have been correlated with defined pathological states such as cancer, their specificity cannot be assured [
24]. Indeed, the same molecules can be activated in multiple pathological conditions and released in EVs. These molecules are usually involved in common cellular processes, such as proliferation, cell death, migration, and so on [
24]. Clinical specificity can be improved by the generation of complex panels of EV-associated biomarkers in which EV number, phenotype, and molecular content are combined and correlated to specific diseases.
Although heterogeneity of EVs can be another limit in their use as biomarkers, their ability to reflect cellular complexity can allow monitoring of a pathological state in all aspects of its progression [
133]. At the same time, a growing number of technologies granting the analysis of specific EV subpopulations have been developed [
134,
135]. These new techniques focus on the improvement of EV yield and purity, trying to eliminate contaminants accounting for the modification of the chemical/biological composition of EVs, as well as their physical properties [
136].
7. Conclusions
EVs are active biological agents that play an essential role in intercellular communication, showing beneficial or detrimental effects on recipient cells based on their origin. The potential of EVs to mirror changes in different compartments of the nephron and their release in the urine emphasizes their role in the pathophysiology of kidney-related diseases. Moreover, uEVs provide a protected microenvironment for molecular content that can be easily screened, searching for stable biomarkers for personalized therapies. Despite the observation that vesicular biomarkers can be more reliable and better correlated with clinical parameters than non-vesicular biomarkers, a critical evaluation of the use of uEVs in kidney-related diseases should be considered. Variability of the isolation methods, the ambiguity of EV classification, inexperience regarding the best EV sources and the existence of misleading factors affecting EV content independently from the kidney pathology are many of the elements that should be considered for future study and application of uEVs in clinical settings.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22126507