The current statistics on cancer show that 90% of all human cancers originate from epithelial cells. Breast and prostate cancer are examples of common tumors of epithelial origin that would benefit from improved drug treatment strategies. About 90% of preclinically approved drugs fail in clinical trials, partially due to the use of too simplified in vitro models and a lack of mimicking the tumor microenvironment in drug efficacy testing. This entry focuses on the epithelial cancers, followed by experimental models designed to recapitulate the epithelial tumor structure and microenvironment. A specific focus is to put on novel technologies for cell culture of spheroids, organoids, and 3D-printed tissue-like models, utilizing biomaterials of natural or synthetic origins, and how the models could be utilized for nanotechnology-based drug delivery in the future.
- spheroid
- organoid
- tumor microenvironment (TME)
- extracellular matrix
- biomaterials
- 3D bioprinting
- high-content imaging
- nanotechnology
- epithelial cancer models
1. Introduction
2. In vitro 3D Experimental Models in Cancer Research
In vitro cancer models are the simplified versions in comparison to in vivo models, when studying cancer mechanisms, and the effect of anticancer moieties on tumor growth and progression. Standard 2D cell culture models fail to recapitulate the cellular mechanisms involved in tumor progression such as cell-cell adhesion, polarization, epithelial differentiation, mechanotransduction, invasion and proper signaling of cells within the tumor tissues. Recent developments have shown that 3D in vitro models have tremendous potential in cancer research due to their most promising characteristic of very closely mimicking the in vivo model systems. An ideal in vitro tumor model should be able to recapitulate the 3D in vivo environment along with reproducing the interaction between tumor and stromal cells, thus regulating the cellular functions. Depending upon the method of cell seeding, the 3D in vitro models could be categorized as scaffold-based and scaffold free models. The scaffold-based models utilize the prefabricated ECMs prepared from different materials such as natural or synthetic materials, or decellularized ECM. While in scaffold-free models, cells proliferate as non-adherent floaters without any support material and 3D constructs are formed due to cellular self-assembly [19].
Non-adherent 3D spheroids can to some degree mimic the solid tumor architecture and it is comprised of different cell layers. The core is composed of necrotic cells while the middle layer has mostly senescent cells. The necrotic or senescent cells of the inner layer is dedicated to the absence or deprivation of nutrients and hypoxic environment, which results in the accumulation of lactate in the spheroids same as that of in vivo solid tumors. The outer layer is formed of cells with high proliferating rates due to convenient access to oxygen and nutrients [20][21].
Tumor cells can also be cultured embedded in ECM, where they spontaneously form 3D structures of organotypic nature, which can be called spheroids if they are round or tumoroids if they have an invasive appearance. Here, single cells that are embedded into ECM grow into multicellular, organotypic structures. Each of the functional structures are of clonal nature, but often has characteristic phenotypes that correspond to different tumor stages. Normal epithelial cells or non-aggressive cancer cells can form well differentiated, polarized, round spheroids with functional basement membranes. In contrast, tumoroids formed by aggressive cells mainly result in undifferentiated clusters of cells, or massive invasive structures. In epithelial cancers, invasion through the ECM is typically of the collective type, and ameoeboid invasion is less frequently observed [22][23]. Tumor cells can also be embedded together with stromal cells and be co-cultured in the ECM. Incorporation of stromal cells such as CAFs will promote genuine, functional interactions between the different cell types, which can be observed in vivo [24]. 3D organotypic cell cultures can therefore act as a bridge between traditional 2D cell culture and costly animal models.
Organoids are more advanced 3D in vitro multicellular structures that mimic the corresponding architecture of in vivo organs. The term organoid is mostly used to describe structures obtained in 3D culture derived from stem cells that are isolated from primary patient samples. The complexity of an organoid is regulated by the developmental potential of the starting stem cells [25]. The organoids can like the spheroids be cultured in non-adherent conditions or embedded in ECM. Organoids are mostly used for translational epithelial research, patient specific treatment planning and disease modelling due to close resemblance to the native tissue composition. However, the 3D organoid culture is advantageous over 3D spheroids due to enhanced physiological and clinical functions. The 3D organoid models of various tumor types have provided concrete evidence to validate the use of these models [26][27][28][29][30]. Thus, in the future, with continuous development, they can provide substantial information in cancer research.
3. 3D Bioprinting Technologies
3.1. Types of 3D Printing Technologies
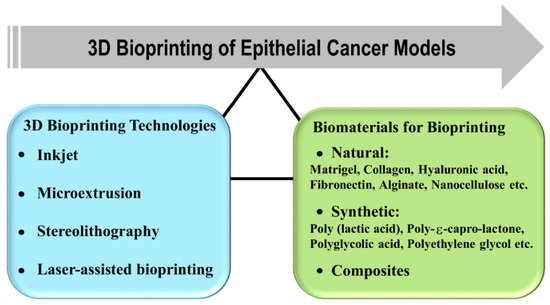
4. Biomaterials for Organotypic 3D Cancer Models
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms22126225
References
- Freddie Bray; Jacques Ferlay Me; Isabelle Soerjomataram; Rebecca L. Siegel; Lindsey A. Torre; Ahmedin Jemal; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018, 68, 394-424, 10.3322/caac.21492.
- Sukumar, S.; McKenzie, K.; Chen, Y. Animal models for breast cancer. Mutat. Res. 1995, 333, 37–44.
- Ittmann, M.; Huang, J.; Radaelli, E.; Martin, P.; Signoretti, S.; Sullivan, R.; Simons, B.W.; Ward, J.M.; Robinson, B.D.; Chu, G.C.; et al. Animal models of human prostate cancer: The consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res. 2013, 73, 2718–2736.
- Hou, J.; Li, L.; Zhu, H.; Chen, H.; Wei, N.; Dai, M.; Ni, Q.; Guo, X. Association between breast cancer cell migration and radiosensitivity in vitro. Oncol. Lett. 2019, 18, 6877–6884.
- Froehlich, K.; Haeger, J.D.; Heger, J.; Pastuschek, J.; Photini, S.M.; Yan, Y.; Lupp, A.; Pfarrer, C.; Mrowka, R.; Schleussner, E.; et al. Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols. J. Mammary Gland Biol. Neoplasia 2016, 21, 89–98.
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843.
- Friedrich, J.; Ebner, R.; Kunz-Schughart, L.A. Experimental anti-tumor therapy in 3-D: Spheroids--old hat or new challenge? Int J Radiat Biol 2007, 83, 849–871.
- Clevers, H.; Tuveson, D.A. Organoid Models for Cancer Res.earch. Annu. Rev. Cancer Biol. 2019, 3, 223–234.
- Vela, I.; Chen, Y. Prostate cancer organoids: A potential new tool for testing drug sensitivity. Expert Rev. Anticancer Ther. 2015, 15, 261–263.
- Gilazieva, Z.; Ponomarev, A.; Rutland, C.; Rizvanov, A.; Solovyeva, V. Promising Applications of Tumor Spheroids and Organoids for Personalized Medicine. Cancers 2020, 12, 2727.
- Fong, E.L.; Wan, X.; Yang, J.; Morgado, M.; Mikos, A.G.; Harrington, D.A.; Navone, N.M.; Farach-Carson, M.C. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 2016, 77, 164–172.
- Lin, D.; Wyatt, A.W.; Xue, H.; Wang, Y.; Dong, X.; Haegert, A.; Wu, R.; Brahmbhatt, S.; Mo, F.; Jong, L.; et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014, 74, 1272–1283.
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12.
- Godugu, C.; Patel, A.R.; Desai, U.; Andey, T.; Sams, A.; Singh, M. AlgiMatrix based 3D cell culture system as an in-vitro tumor model for anticancer studies. PLoS ONE 2013, 8, e53708.
- Aggarwal, B.B.; Danda, D.; Gupta, S.; Gehlot, P. Models for prevention and treatment of cancer: Problems vs promises. Biochem Pharm. 2009, 78, 1083–1094.
- Mao, S.; Pang, Y.; Liu, T.; Shao, Y.; He, J.; Yang, H.; Mao, Y.; Sun, W. Bioprinting of in vitro tumor models for personalized cancer treatment: A review. Biofabrication 2020, 12, 042001.
- Beri, P.; Matte, B.F.; Fattet, L.; Kim, D.; Yang, J.; Engler, A.J. Biomaterials to model and measure epithelial cancers. Nat. Rev. Mater. 2018, 3, 418–430.
- Härmä, V.; Virtanen, J.; Makelä, R.; Happonen, A.; Mpindi, J.P.; Knuuttila, M.; Kohonen, P.; Lotjonen, J.; Kallioniemi, O.; Nees, M. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS ONE 2010, 5, e10431.
- Weijie Peng; Pallab Datta; Bugra Ayan; Veli Ozbolat; Donna Sosnoski; Ibrahim T. Ozbolat; 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomaterialia 2017, 57, 26-46, 10.1016/j.actbio.2017.05.025.
- Andrew I. Minchinton; Ian F. Tannock; Drug penetration in solid tumours. Nature Cancer 2006, 6, 583-592, 10.1038/nrc1893.
- Olivier Tredan; Carlos M. Galmarini; Krupa Patel; Ian F. Tannock; Drug Resistance and the Solid Tumor Microenvironment. Journal of the National Cancer Institute 2007, 99, 1441-1454, 10.1093/jnci/djm135.
- Ville Härmä; Johannes Virtanen; Rami Mäkelä; Antti Happonen; John-Patrick Mpindi; Matias Knuuttila; Pekka Kohonen; Jyrki Lötjönen; Olli Kallioniemi; Matthias Nees; et al. A Comprehensive Panel of Three-Dimensional Models for Studies of Prostate Cancer Growth, Invasion and Drug Responses. PLoS ONE 2010, 5, e10431, 10.1371/journal.pone.0010431.
- Ville Härmä; Hannu-Pekka Schukov; Antti Happonen; Ilmari Ahonen; Johannes Virtanen; Harri Siitari; Malin Åkerfelt; Jyrki Lötjönen; Matthias Nees; Quantification of Dynamic Morphological Drug Responses in 3D Organotypic Cell Cultures by Automated Image Analysis. PLOS ONE 2014, 9, e96426, 10.1371/journal.pone.0096426.
- Malin Åkerfelt; Neslihan Bayramoglu; Sean Robinson; Mervi Toriseva; Hannu-Pekka Schukov; Ville Härmä; Johannes Virtanen; Raija Sormunen; Mika Kaakinen; Juho Kannala; et al. Automated tracking of tumor-stroma morphology in microtissues identifies functional targets within the tumor microenvironment for therapeutic intervention. Oncotarget 2015, 6, 30035-30056, 10.18632/oncotarget.5046.
- Natalie De Souza; Organoids. Nature Methods 2018, 15, 23-23, 10.1038/nmeth.4576.
- Camilla Calandrini; Frans Schutgens; Rurika Oka; Thanasis Margaritis; Tito Candelli; Luka Mathijsen; Carola Ammerlaan; Ravian L. Van Ineveld; Sepide Derakhshan; Sanne De Haan; et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nature Communications 2020, 11, 1-14, 10.1038/s41467-020-15155-6.
- Norman Sachs; Joep De Ligt; Oded Kopper; Ewa Gogola; Gergana Bounova; Fleur Weeber; Anjali Vanita Balgobind; Karin Wind; Ana Gracanin; Harry Begthel; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373-386.e10, 10.1016/j.cell.2017.11.010.
- Yoshiaki Maru; Naotake Tanaka; Makiko Itami; Yoshitaka Hippo; Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecologic Oncology 2019, 154, 189-198, 10.1016/j.ygyno.2019.05.005.
- Jumpei Kondo; Masahiro Inoue; Application of Cancer Organoid Model for Drug Screening and Personalized Therapy. Cells 2019, 8, 470, 10.3390/cells8050470.
- Ilmari Ahonen; Malin Åkerfelt; Mervi Toriseva; Eva Oswald; Julia Schüler; Matthias Nees; A high-content image analysis approach for quantitative measurements of chemosensitivity in patient-derived tumor microtissues. Scientific Reports 2017, 7, 6600, 10.1038/s41598-017-06544-x.
- Zhang, Y.S.; Duchamp, M.; Oklu, R.; Ellisen, L.W.; Langer, R.; Khademhosseini, A. Bioprinting the Cancer Microenvironment. Acs Biomater. Sci. Eng. 2016, 2, 1710–1721.
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006, 1, 910–917.
- Cohen, D.L.; Malone, E.; Lipson, H.; Bonassar, L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006, 12, 1325–1335.
- Zhou, X.; Tenaglio, S.; Esworthy, T.; Hann, S.Y.; Cui, H.; Webster, T.J.; Fenniri, H.; Zhang, L.G. Three-Dimensional Printing Biologically Inspired DNA-Based Gradient Scaffolds for Cartilage Tissue Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 33219–33228.
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B.; Lopez, J.; Faucon, M.; Pippenger, B.; Bareille, R.; Remy, M.; Bellance, S.; et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010, 6, 2494–2500.
- Khalil, S.; Sun, W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater. Sci. Eng. C 2007, 27, 469–478.
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. Part A 2013, 101, 272–284.
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater 2011, 98, 160–170.
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Eng. Part A 2008, 14, 41–48.
- Wang, X.; Wang, Q.; Xu, C. Nanocellulose-Based Inks for 3D Bioprinting: Key Aspects in Research Development and Challenging Perspectives in Applications-A Mini Review. Bioengineering 2020, 7, 40.
- Langer, E.M.; Allen-Petersen, B.L.; King, S.M.; Kendsersky, N.D.; Turnidge, M.A.; Kuziel, G.M.; Riggers, R.; Samatham, R.; Amery, T.S.; Jacques, S.L.; et al. Modeling Tumor Phenotypes In Vitro with Three-Dimensional Bioprinting. Cell Rep. 2019, 26, 608–623.e606.
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 2011, 6, 204–212.
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed Mater. Res. Part A 2013, 101, 1255–1264.
- Della Bona, A.; Cantelli, V.; Britto, V.T.; Collares, K.F.; Stansbury, J.W. 3D printing restorative materials using a stereolithographic technique: A systematic review. Dent. Mater. 2021, 37, 336–350.
- Zhou, X.; Esworthy, T.; Lee, S.J.; Miao, S.; Cui, H.; Plesiniak, M.; Fenniri, H.; Webster, T.; Rao, R.D.; Zhang, L.G. 3D Printed scaffolds with hierarchical biomimetic structure for osteochondral regeneration. Nanomedicine 2019, 19, 58–70.
- Zhou, X.; Nowicki, M.; Cui, H.T.; Zhu, W.; Fang, X.Q.; Miao, S.D.; Lee, S.J.; Keidar, M.; Zhang, L.J.G. 3D bioprinted graphene oxide-incorporated matrix for promoting chondrogenic differentiation of human bone marrow mesenchymal stem cells. Carbon 2017, 116, 615–624.
- Dinca, V.; Kasotakis, E.; Catherine, J.; Mourka, A.; Ranella, A.; Ovsianikov, A.; Chichkov, B.N.; Farsari, M.; Mitraki, A.; Fotakis, C. Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett. 2008, 8, 538–543.
- Colina, M.; Serra, P.; Fernandez-Pradas, J.M.; Sevilla, L.; Morenza, J.L. DNA deposition through laser induced forward transfer. Biosens. Bioelectron 2005, 20, 1638–1642.
- Hopp, B.; Smausz, T.; Kresz, N.; Barna, N.; Bor, Z.; Kolozsvari, L.; Chrisey, D.B.; Szabo, A.; Nogradi, A. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2005, 11, 1817–1823.
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515.
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18.
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes. Dis. 2017, 4, 185–195.
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976.
- Wang, F.; Weaver, V.M.; Petersen, O.W.; Larabell, C.A.; Dedhar, S.; Briand, P.; Lupu, R.; Bissell, M.J. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA 1998, 95, 14821–14826.
