Age related macular degeneration (AMD) is a disease associated with aging of the central area of the retina called the macula. It results in a progressive loss of central vision.
In western countries, AMD is the leading cause of severe vision loss in people over the age of 50. About 25 to 30 million people suffer from AMD. Due to the aging of the population, this figure could double in the next 35 years.
- Age related macular degeneration (AMD)
- angiogenesis
- treatment of AMD
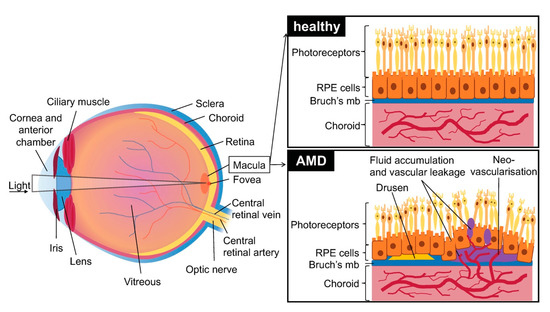
vAMD is more aggressive than the dry form. Currently, the only treatment relies on intravitreal injections of anti-VEGF to inhibit angiogenesis and minimize visual loss.
Three main drugs are currently approved: aflibercept (Eylea, a fusion protein of the extracellular domains of VEGFR1-2 serving as decoy receptors for VEGF, VEGFB and PlGF), bevacizumab (Avastin, a recombinant humanized monoclonal antibody that inhibits VEGF), and ranibizumab (Lucentis, a humanized monoclonal antibody fragment derived from the same parent antibody than bevacizumab). Both ranibizumab and bevacizumab have similar efficacy in randomized trials over a period of 24 months.[9],[10]
Injections are performed monthly for three months, and then every two months. Once there is a reduction in symptoms, each patient is followed individually in order to reduce the number of injections needed and to treat on a case to case basis. In a “treat-and-extend” regimen, monitoring visits are performed to fine tune the treatment and injections are performed during the visits. This method reduces the number of visits and the number of injections, reducing costs and the strain on patients which are often reluctant to undergo multiple injections.
However, frequent intravitreal injections of anti-VEGF drugs are associated with ocular hypertension, retinal detachment, ocular infection, and poor patient compliance. Moreover, repeated injections can sometimes lead to intra ocular inflammation, infectious endophthalmitis, or RPE tearing.[9,11]
A novel inhibitor called conbercept (Lumitin) developed by the Chengdu Kang Hong Biotech is currently in phase 3 clinical trials. Conbercept binds to several VEGF family members including VEGF-A, VEGF-B, and PlGF. It has a longer half-life and a better bioavailability than ranibizumab or aflibercept. It only needs quarterly administrations, thus reducing the load on patients and the healthcare system.[12]
Other means of treating vAMD were used before the widespread of anti-VEGF injections. However these methods were less reliable with relapses in following years and either did not improve visual recovery. They include laser photocoagulation, which consists of laser treatment in the extra-foveal, juxta-foveal or sub-foveal zone; and photodynamic therapy, which consists of a non-thermal laser treatment. A photosensitizer is injected in the area of interest and activated by a specific light wave. This method prevents the occurrence of thermal tissue damage.[13]
Finally, vitamin supplements in the intermediate stages of the disease may delay the development of AMD in the other eye and the reduction of vision loss; specifically vitamins E, C, carotenoids, and mineral supplementation (zinc oxide and cupric oxide).[13]
The procedure of anti-VEGF treatment is still quite time consuming and traumatic for the patients. In addition, even if anti-VEGF treatments delays vision loss caused by vAMD, many patients nevertheless remain refractory to these treatments or become resistant. It is therefore absolutely necessary to develop new therapeutic strategies to treat patients in the first and second line. Thus, it is very important to implement different models of vAMD in order to reach this objective.
Experimental models of vAMD are essential to screen different innovative therapeutics. The currently used in vitro and in vivo models in ophthalmic translational research and their relevance are discussed in this review [14].
References
1. Gheorghe, A.; Mahdi, L.; Musat, O. AGE-RELATED MACULAR DEGENERATION. Rom J Ophthalmol 2015, 59, 74–77.
2. Imamura, Y.; Noda, S.; Hashizume, K.; Shinoda, K.; Yamaguchi, M.; Uchiyama, S.; Shimizu, T.; Mizushima, Y.; Shirasawa, T.; Tsubota, K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proceedings of the National Academy of Sciences 2006, 103, 11282–11287, doi:10.1073/pnas.0602131103.
3. Kaarniranta, K.; Tokarz, P.; Koskela, A.; Paterno, J.; Blasiak, J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol Toxicol 2017, 33, 113–128, doi:10.1007/s10565-016-9371-8.
4. Fleckenstein, M.; Mitchell, P.; Freund, K.B.; Sadda, S.; Holz, F.G.; Brittain, C.; Henry, E.C.; Ferrara, D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018, 125, 369–390, doi:10.1016/j.ophtha.2017.08.038.
5. Ferrington, D.A.; Sinha, D.; Kaarniranta, K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Progress in Retinal and Eye Research 2016, 51, 69–89, doi:10.1016/j.preteyeres.2015.09.002.
6. Yu, Y.; Bhangale, T.R.; Fagerness, J.; Ripke, S.; Thorleifsson, G.; Tan, P.L.; Souied, E.H.; Richardson, A.J.; Merriam, J.E.; Buitendijk, G.H.S.; et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Human Molecular Genetics 2011, 20, 3699–3709, doi:10.1093/hmg/ddr270.
7. Seddon, J.M.; Yu, Y.; Miller, E.C.; Reynolds, R.; Tan, P.L.; Gowrisankar, S.; Goldstein, J.I.; Triebwasser, M.; Anderson, H.E.; Zerbib, J.; et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat. Genet. 2013, 45, 1366–1370, doi:10.1038/ng.2741.
8. van de Ven, J.P.H.; Nilsson, S.C.; Tan, P.L.; Buitendijk, G.H.S.; Ristau, T.; Mohlin, F.C.; Nabuurs, S.B.; Schoenmaker-Koller, F.E.; Smailhodzic, D.; Campochiaro, P.A.; et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat Genet 2013, 45, 813–817, doi:10.1038/ng.2640.
9. Al-Zamil, W.; Yassin, S. Recent developments in age-related macular degeneration: a review. CIA 2017, Volume 12, 1313–1330, doi:10.2147/CIA.S143508.
10. Kodjikian, L.; Souied, E.H.; Mimoun, G.; Mauget-Faÿsse, M.; Behar-Cohen, F.; Decullier, E.; Huot, L.; Aulagner, G. Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology 2013, 120, 2300–2309, doi:10.1016/j.ophtha.2013.06.020.
11. Ghasemi Falavarjani, K.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye 2013, 27, 787–794, doi:10.1038/eye.2013.107.
12. Liu, K.; Song, Y.; Xu, G.; Ye, J.; Wu, Z.; Liu, X.; Dong, X.; Zhang, M.; Xing, Y.; Zhu, S.; et al. Conbercept for Treatment of Neovascular Age-related Macular Degeneration: Results of the Randomized Phase 3 PHOENIX Study. American Journal of Ophthalmology 2019, 197, 156–167, doi:10.1016/j.ajo.2018.08.026.
13. Hubschman, J.P.; Reddy, S.; Schwartz, S.D. Age-related macular degeneration: current treatments. Clin Ophthalmol 2009, 3, 155–166, doi:10.2147/opth.s2094.
14. Rastoin, O.; Pagès, G.; Dufies, M. Experimental Models in Neovascular Age Related Macular Degeneration. Int J Mol Sci. 2020 Jun 29;21(13). doi:10.3390/ijms21134627.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21134627
