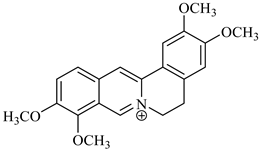
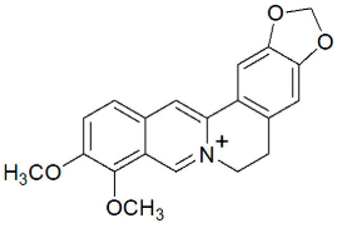
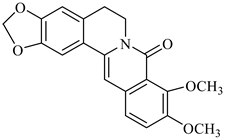
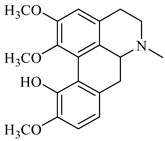
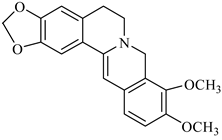
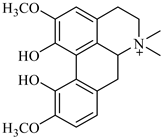
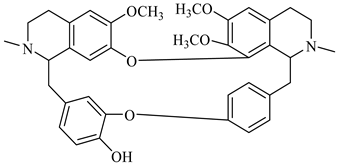
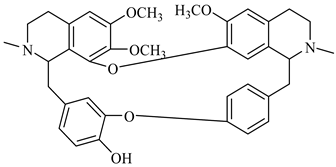
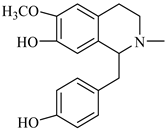
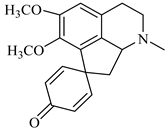
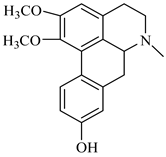
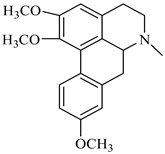
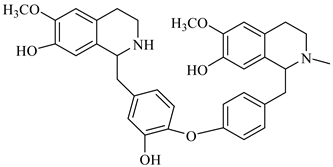
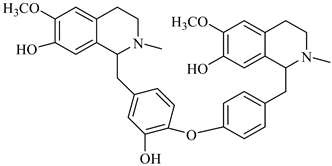
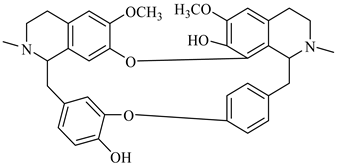
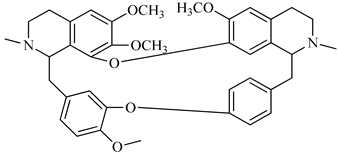
The genus Berberis includes about 500 different species and commonly grown in Europe, the United States, South Asia, and some northern areas of Iran and Pakistan. Leaves and fruits can be prepared as food flavorings, juices, and teas. Phytochemical analysis of these species has reported alkaloids, tannins, phenolic compounds and oleanolic acid, among others. Moreover, p-cymene, limonene and ocimene as major compounds in essential oils were found by gas chromatography. Berberis is an important group of the plants having enormous potential in the food and pharmaceutical industry, since they possess several properties, including antioxidant, antimicrobial, anticancer activities.
- Berberis
- food preservative
- alkaloid
- antioxidant
- human health
1. Introduction
2. Berberis Plants Essential Oils and Phytochemical Composition
|
Chemical structure |
Name |
Plant |
|---|---|---|
|
|
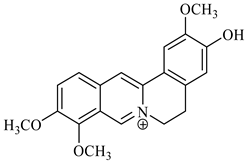
palmatine |
B. vulgaris |
|
|
berberine |
B. vulgaris |
|
|
oxyberberine |
B. vulgaris |
|
|
isocoridine |
B. vulgaris |
|
|
lambertine |
B. vulgaris |
|
|
magniflorine |
B. vulgaris |
|
|
oxycanthine |
B. vulgaris |
|
|
berbamine |
B. aristata |
|
|
(+)-N-methylcoclaurine |
B. montana |
|
|
(−)-pronuciferine |
B. montana |
|
|
(+)-9-hydroxynuciferine |
B. montana |
|
|
(+)-orientine |
B. montana |
|
|
2-norberbamunine |
B. stoloniferais |
|
|
berbamunine |
B. stoloniferais |
|
|
aromoline |
B. stoloniferais |
|
|
isotetrandrine |
B. stoloniferais |
|
|
jatrorrhizine |
B. umbellate |
3. Food Preservative Applications of Berberis Plants
|
S. No. |
Species |
Part |
Country |
Extract/Model/Compound |
Tested Micro-Organism |
Results |
Reference |
|---|---|---|---|---|---|---|---|
|
1 |
B. aristata |
Stem and leaves |
Nepal |
Hexane, Ethyl acetate, Methanol |
Staphylococcus aureus, Kleibsella pneumoniae, Salmonella typhimurium |
Against S. aureus: methanol significant zone of inhibition (21 mm), ethyl acetate extracts moderate activity, hexane extract of stem slightly active. |
[73] |
|
2 |
B. aristata, and B. ligulata |
Bark stem Leaves |
Nepal |
Ethanol |
Bacillus subtilis, Escherichia coli, Pseudomona aeruginosa, Salmonella. typhi, Salmonella dyjenteriae, Salmonella cholerae |
Ethanol extract of B. aristata: largest zone of inhibition (21 mm) against B. subtilis and the smallest MBC value (90 mg/mL) for S. aureus. Gram positive bacteria more susceptible to the ethanol extract. B. aristata relatively broad-spectrum antibacterial activity. |
[74] |
|
3 |
B. vulgaris |
Stem |
Iran |
Ethanol |
P. aeruginosa, Acinetobacter baumannii, E. coli and Salmonella enteritidis |
MIC determination: stem extracts inhibit the growth of all the studied bacteria (3900 to 37,500 μg/mL) by synergistic effects with ciprofloxacin. |
[75] |
|
4 |
B. asiatica |
Leaves |
Uttarakhand, India |
Methanol |
E. coli, Enterobacter aerogenes, Proteus vulgaris, P. aeruginosa, K. pneumoniae, B. subtilis, S. aureus |
Methanol extracts of leaves: high inhibitory potential on S. aureus, K. pneumoniae, E. coli, B. subtilis and P. vulgaris in all concentration. |
[76] |
|
5 |
B. aristata, B. asiatica, B. lycium |
Stem |
Bangalore, India |
Methanol |
Nocardia sp., S. aureus, S. pneumonia, P. aeruginosa, Streptococcus viridians, E. coli |
Sensitivity to Nocardia sp., S. pneumonia and E. coli. |
[77] |
|
6 |
B. glaucocarpa |
Root wood |
Pakistan |
Ethanol |
SMRSA, EMRSA, Mycobacterium marinum, E. coli, Trypanosoma brucei |
Berberine (MIC = 12.5 and 25 μg/mL), berberine chloroform (MIC = 25 and 12.5 μg/mL) and syringaresinol (12.5 μg/mL): very active against SMRSA, M. marinum and T. brucei. |
[78] |
|
7 |
B. vulgaris |
Stem bark |
Romania |
Ethanol |
Botrytis cinerea |
B. vulgaris bark extract, berberine, and fluconazole significantly inhibited growth of B. cinerea. |
[79] |
|
8 |
B. vulgaris |
Ethanol |
S. aureus, Staphylococcus epidermidis, K. pneumoniae, B. subtilis, E. coli, Aspergillus niger, Trichoderma, Alternaria solanai |
20 mm zone of inhibition against E. coli. Good activity against B. Subtilis, moderate against Trichoderma, insignificant against other stains. |
[80] | ||
|
9 |
B. vulgaris and its active constituent, berberine |
Root |
Egypt |
Ethanolic extract |
Candida albicans, E. coli |
Berberis ethanolic extract and berberine standard can inhibit C. albicans and E. coli growth. |
[81] |
|
10 |
B. vulgaris |
Fruit |
Pakistan |
Distilled water |
S. aureus, Proteus, S. typhi, Salmonella paratyphi A, Salmonella paratyphi B, K. pneumoniae, E. coli, P. aeruginosa |
Antibacterial activity against all tested pathogens. |
[82] |
|
11 |
B. thunbergii |
Fruit |
Hungary |
Juice; water extract and -methanol extract |
B. subtilis, Bacillus cereus var. mycoides, E. coli, Serratia marcescens |
Juice, water extract and methanol extract showed activity against all bacteria. |
[83] |
|
12 |
B. calliobotrys |
Stems and branches |
Pakistan |
Methanol |
B. subtilis, P. aeruginosa, S. aureus fungal strains namely C. albicans, Penicillium notatum |
The methanol extract, ethyl acetate and n-butanol fractions: maximum zone of inhibition against all bacterial strains especially S. aureus and antifungal effects. |
[84] |
|
13 |
B. lycium |
Roots |
Libya |
Distilled water, ethanol, isopropanol and methanol |
Pseudomonas sp., E. coli, Streptococcus sp., Staphylococcus sp. |
Methanolic displayed maximum inhibitory zone (16 mm), isopropanol extract (13 mm) and ethanol extract (12 mm). The aqueous extract exhibited the least inhibitory zone (10 mm). The methanolic extract: maximum inhibitory zone (12 mm), Pseudomonas (11 mm) and Staphylococcus (10 mm). |
[85] |
|
14 |
B. hispanica |
Root Bark |
Marocco |
Ethanolic extract |
Mycobactérium smegmatis, Mycobacterium aurum |
The ethanolic extract from root bark displayed an important antimycobacterial activity. The inhibition zones for M. aurum A+ were significantly larger than those for M. smegmatis MC2. |
[86] |
|
15 |
B. ruscifolia |
- |
Argentina |
Acetone, chloroform-methanol (1:1) and methanol |
E. coli, P. aeruginosa, Listeria monocytogenes, S. aureus |
All extracts exhibited antibacterial activity with MIC varying from 16 to 2 mg/mL. The highest inhibition with acetonic and chloroform-methanolic extracts of species against S. aureus (MIC = 2 mg/mL). Methanolic extracts B. ruscifolia showed no antibacterial activity against all tested bacteria. |
[87] |
|
16 |
B. aristata |
Stem bark |
India |
Ethanol and aqueous extracts |
Shigella flexneri, Shigella sonnei, Shigella dysenteriae, Shigella boydii |
Extracts of B. aristata: antibacterial activity against four strains of Shigella (8 and 23 mm). |
[88] |
|
17 |
B. aristata, B. asiatica, B. chitria and B. lycium |
Root and stem |
India |
Ethanol |
Micrococcus luteus, B. subtilis, B. cereus, Enterobacter aerogenus, E. coli, K. pneumoniae, Proteus mirabilis, P. aeruginosa, S. aureus, S. typhimurium, Streptococcus pneumonia, Fungal strains Aspergillus nidulans, C. albicans, Aspergillus terreus, Trichophyton rubrum, Cistus albidus, Aspergillus flavus, A. niger |
B. lycium, B. aristata and B. asiatica root extract showed significant antifungal activity against A. terreus and A. flavus. B. aristata root and B. lycium (stem) extracts gave very low MIC values (0.31 μg/mL) as compared to other tested species. |
[89] |
|
18 |
B. Lycium |
Root |
Pakistan |
Ethanol, petroleum ether |
S. aureus, S. epidermidis, B. subtilis, S. typhi, E. coli, C. albicans |
The ethanolic and aqueous crud root extract: most effective antifungal and antibacterial agents. |
[90] |
|
19 |
B. integerrima Syn: B. densiflora |
Roots |
Iran |
Methanol |
Brucella abortus |
MIC and MBC results, jatrorhizine exhibited higher antibacterial activity with MIC (0.78 μg/mL) and MBC (1.56 μg/mL) compared with the standard (streptomycin, 10 μg/mL). |
[91] |
|
20 |
B. lycium |
Roots |
Pakistan |
Hydric extract |
E. coli, Pseudomonas, Staphylococcus, Proteus |
Significant activity against E. coli and Proteus (80 to 100%), while it demonstrated a good activity against Pseudomonas and Staphylococcus (60 to 70%). |
[92] |
|
21 |
B. aristata |
Bark and leaves |
India |
Methanol, ethanol and hexane |
B. subtilis, Agrobacterium tumefaciens, E. coli, Xanthomonas. Phaseoli, Erwinia chrysanthemi |
All the extracts of tested plants showed variable activity against all the tested bacterial strains. Methanol extract revealed highest antibacterial activity (11 mm) recorded against E. chrysanthemi. Hexane extract: totally inactive against all the tested strains. |
[93] |
|
22 |
B. aristata |
Roots |
India |
Aqueous and alcohol extracts |
S. aureus, B. subtilis, E. coli, S. typhimurium |
Alcoholic and aqueous extract showed antimicrobial activity against four tested bacteria. B. aristata exhibited highest zone of inhibition for B. subtilis followed by S. aureus, E. coli and S. typhimurium. |
[94] |
|
23 |
B. microphylla |
Leaves, stems and roots |
Chile |
Methanol |
E. coli, S. typhimurium, L. monocytogenes, E. aerogenes, S. aureus, B. cereus, S. epidermidis and B. subtilis |
All extract possesses significant antibacterial activity against Gram-positive bacteria but not against Gram-negative bacteria. |
[95] |
|
24 |
B. lycium |
Root bark |
Pakistan |
E. coli, K. pneumoniae, P. aeruginosa, S. aureus, B. subtilis |
Silver nanoparticles were very active against Gram-negative and Gram-positive bacteria Aqueous bark extract (10 μg/mL) possess highest activity against E. coli and P. aeruginosa. |
[96] | |
|
25 |
B. vulgaris |
Fruit |
Iran |
L. monocytogenes |
Average diagonal of growing area in disk diffusion test for species: 12 mm and MIC was 125 μg/mL and MBC of B. vulgaris was 500 μg/mL. |
[97] | |
|
26 |
B. aristata |
Stem bark |
Alcohol |
In vivo in an animal model using Sprague Dawley rats |
Carbapenem-resistant E. coli |
An aquo-alcoholic extract of the species: effectively manage peritonitis induced by Carbapenem-resistant E. coli in a rat model at a single post-exposure prophylactic dose of 0.5 mg/kg body weight. |
[98] |
|
27 |
B. aristata |
Roots |
India |
Aqueous and alcoholic extract of fresh roots, as well as aqueous extract of dried roots |
S. aureus, S. epidermidis, Streptococcus pyogenes, Streptococcus viridans, Enterococcus faecalis, B. subtilis, B. cereus, E. coli, K. pneumoniae, P. aeruginosa, P. vulgaris, P. mirabilis, S. typhi, S. paratyphi A, S. typhimurium, S. dysenteriae type 1, Vibrio cholerae |
All three extracts displayed wide antibacterial activity against Gram-positive bacteria. Among the Gram-negative bacteria tested, the antibacterial activity was limited to E. coli, S. typhimurium, S. dysenteriae type 1 and V. cholerae. All extracts also possess antifungal activity against the fungal species tested, except Candida krusei. |
[99] |
|
28 |
B. aristata |
Root Stem Leaf |
Pakistan |
E. coli, S. typhi, S. aureus, Shigella, Citrobacter, P. vulgaris,Enterobacter, Streptococcus pyrogenes, V. cholera, Klebsiella spp., A. niger, Cladosporium, Rhizoctonia, Alternaria, Trichoderma, Penicillium, Curvularia, Paecilomyces and Rhizopus |
The extracts significantly inhibited the growth of the studied microbes, except A. niger, Curvularia, Paecilomyces and Rhizopus. |
[100] | |
|
29 |
B. aristata |
India |
V. cholerae, S. aureus |
All the strains of V. cholerae are susceptible. All the Salmonella sp., Pseudomonas sp., and some of the E. coli strains are highly resistant, except some strains of E. coli as AL26, and Shigella sp. are susceptible. All Xanthomonas sp. were highly susceptible. Berberine sulfate showed antifungal action against C. albicans, Candida tropicalis, Trichophyton mentagrophytes, Microsporum gypseum, Cryptococcus neoformans and Sporothrix schenkii, Mycobacterium tuberculosis var. hominis H37RV and Entamoeba histolytica. |
[101] | ||
|
30 |
B. heterophylla |
Leaves, stems and roots berberine |
Argentina |
S. aureus, E. faecali, P.aeruginosa, E. coli, C. albicans, Candida glabrata, Candida haemulonii, Candida lusitaniae, C. krusei, Candida parapsilosis |
The aqueous extracts of B. heterophylla do not possess significant antimicrobial activity. Berberine displayed a significant antibacterial and antifungal activity against S. aureus and different Candida spp., some of them obtained from the clinical isolated. |
[102] | |
|
31 |
B. amurensis |
Branches and leaves |
Korea |
Bacillus atrophaeus, Kocuria rhizophila, M. luteus, S. epidermidis, B. subtilis subsp. Spizizenii, K. pneumoniae, Enterobacter cloacae, Salmonella enterica subsp. enterica, P. aeruginosa |
No significant activity against gram-negative bacteria. |
[103] | |
|
32 |
B. croatica and B. vulgaris |
Roots, leaves, and twigs |
Croatia |
Ethanol |
B. subtilis, S. aureus, E. coli, P. aeruginosa, C. albicans |
Extracts of both species: significant antibacterial activity against the Gram-positive bacteria. Root extracts of B. croatica: activity against P. aeruginosa, and leaf extracts against B. subtilis. Neither species possessed antifungal activity. Leaf extracts of B. croatica: antibacterial activity against B. subtilis. Likewise, neither of the species extracts showed activity against E. coli and C. albicans, except when were diluted. Ethanolic extracts of twigs of both species: inactive against B. subtilis and against S. aureus, with the exception of B. croatica twig from Kiza locality. |
[104] |
|
33 |
B. lycium |
Roots |
India |
Hexane extract, Methanolic extract, aqueous extract and berberine |
K. pneumonia, E. coli, P. aeuroginosa, S. aureus, B. subtilis, C. albicans, A. niger, Aspergillus fumigates |
Methanolic extract of species was highly effective against E. coli, S. aureus, B. subtilis, C. albicans, A. fumigates. Pure berberine was effective against E. coli and C. albicans. |
[105] |
|
34 |
B. aetnensis |
Roots |
Italy |
Ethanol ether and chloroform |
S. aureus, B. subtilis, E. faecalis, E. coli, P. aeruginosa, Stenotrophomonas maltophilia, against 14 strains of nosocomial origin: two strains of S. aureus (1 Met-S, 1 Met-R); four strains of S. epidermidis (2 Met-S, 2 Met-R); three strains of E. coli; four strains of P. aeruginosa, Hafnia alvei and C. albicans, C. parapsilosis, C. krusei |
The root and leaf extracts showed a greater activity against Gram-positive bacteria and yeasts than against Gram-negative bacteria, except for P. aeruginosa. The chloroform extract of leaves was more active than the ethanol. |
[106] |
|
35 |
B. thunbergii, B. vulgaris |
Roots |
USA |
E. coli, P. aeruginosa, S. aureus, S. mutans, and S. pyogenes |
Ethanolic extracts more active against studied bacteria, strongest activity against S. pyogenes and S. aureus. |
[107] | |
|
36 |
B. vulgaris |
Root bark |
Algeria |
Methanol and water |
S. aureus, E. faecalis, E. coli, E. cloacae, K. pneumoniae, P. aeruginosa |
The extracts of species root barks presented a strong activity against S. aureus (23.0 mm), a weak activity against E. faecalis (13.0 mm) and no activity toward other strains. |
[108] |
This entry is adapted from the peer-reviewed paper 10.3390/foods8100522