The problem of treating viral infections is extremely important both in connection with the emergence of new viral diseases and in connection with the low efficiency of existing approaches to the treatment of known viral infections. This entry is devoted to the use of porphyrins, chlorins, and phthalocyanines for the fight against viral infections using chemical and photochemical inactivation methods. The purpose of this work is to summarize the main approaches developed to date to chemical and photodynamic inactivation of human and animal viruses using porphyrins and their analogs, as well as to analyze and discuss information on viral targets and antiviral activity of porphyrins, chlorins and their derivatives obtained in the last 10-15 years, in order to identify the most promising areas.
- viruses
- chlorin
- inactivaion
- porphyrins
1. Introduction
Despite their comparative simplicity, viruses are extremely diverse in their genetic material and replication mechanisms [1].
As a rule, the life cycle of a virus is a complex process, which is divided into the following stages:
(1) attachment of the virion to the cell surface (linking of the host cell receptor);
(2) internalization of the virus into the cell (endocytosis);
(3) decapsidation, cytoplasmic transport and nuclear imports;
(4) transcription and replication of viral RNA / DNA;
(5) nuclear export and protein synthesis;
(6) assembly, release of propagated virions from the host cell membrane.
All these stages of the life cycle of a virus are of great importance for its virulence, replication and transmission. Violation of any of these can lead to the development of a potential and effective strategy for controlling and preventing viral infection. The inactivation of the first three stages of the life cycle of the virus has the greatest practical application, so this review will be focused on them.
2. Inactivation at the stage of attachment of the virion to the cell surface (receptor binding) and fusion with the cell membrane
In this part we consider the methods of inactivating viral particles, using porphyrins and their analogues at the first stage of the life cycle, taking as the example human immunodeficiency virus (HIV). The vast majority of studies focus on HIV inactivation, partly because this virus appeared relatively long ago (in 1983) and has been well studied, but to a greater extent, owing to the slowly progressive disease caused by it which often leads to death. According to WHO, there are about 40 million people diagnosed with HIV in the world. The human immunodeficiency virus belongs to the genus lentiviruses (Lentivirus) of the family of retroviruses (Retroviridae).
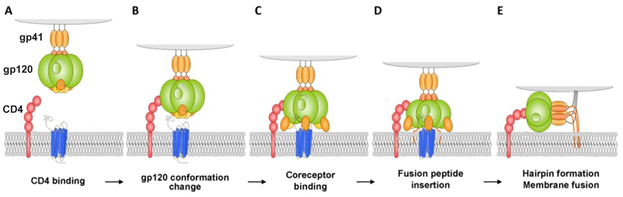
The first stage of attachment of the virion to the surface of the cell and fusion with the cell membrane is determined by the spikes on the surface of the virion. The spikes on the viral particle are formed by Env glycoprotein (envelope protein) which consists of two subunits: the variable protein gp1209 performing the function of binding to the CD4 receptor, and of the more conservative transmembrane gp41, which holds the gp120 molecule in the membrane (Figure 1). The gp120 viral glycoprotein strongly links the CD4 receptor (Figure 1).
Figure 1. Model for HIV-1 entry. (A and B) Binding of Cluster of Differentiation (CD)4 to glycoprotein (gp)120 exposes a coreceptor binding site in gp120; (C and D) Coreceptor binding causes the exposure of the gp41 fusion peptide and its insertion into the membrane of the target cell in a triple-stranded coiled-coil; (E) Formation of a helical hairpin structure in which gp41 folds back on itself is coincident with membrane fusion [2].
As a result of this interaction, gp120 undergoes conformational changes that also provide binding the coreceptor molecule CXCR4 or CCR5 (Figure 1). This is followed by the stage of internalization of the virus into the host cell; the latter ensures the fusion of the cell membranes of the virus and the host due to the gp41 viral transmembrane protein. Thus, the proteins of the gp120 or gp41 virus are the most important and critical targets, since if they are damaged or linked, further propagation of the HIV virus becomes impossible. Among the porphyrins and their analogues many very promising compounds have been identified that are capable of forming strong complexes with the gp120 protein glycoprotein [3], and the complex formed in this way does not allow the viral gp120 of protein to bind to the host cell CD4 receptor [4].
It was found that the porphyrin-zinc complex containing three 4-nitrophenyl groups and one 4-methylpyridinium group in the mesoposition most effectively blocks the penetration of virions (4 μM,> 99%). A cationic substituent is necessary to increase the solubility of P&A in aqueous media, and three nitro groups provide linking of the gp120 protein with the glycoprotein and thereby inhibit fusion between the virus and the cell membrane.
It should be noted that targeted modelling of the P&A structure for linking with a specific region of the target has not yet been carried out. At this stage of development of this research direction, the P&A is mainly screened, both using computer modelling [5] and experimental methods [6]. For example, the antiviral activity of methyl and metal-free porphyrin and chlorine compounds against a wide range of viruses with and without envelope was evaluated in [7]. Chlorophyllide was most effective against hepatitis B virus (HBV). Only 3 μM chlorophyllide reduced HBV DNA signal by 80%. To establish the mechanism of the virucidal activity of chlorophyllide, the authors conducted in vitro biochemical studies in which chlorophyllide was introduced on each life cycle of the virus. It turned out that chlorophyllide changed the structure of the capsid of HBV virions and directly destroyed the HBV virions in the culture medium of producer cells, and it resulted in the loss of DNA virion. Chlorophyllide does not have a noticeable effect on the viability of the host cells and intracellular products of viral genes, it is safe for humans at a dose of 300 mg / day for 4 months [8].
All the studies reviewed above are based on the method of chemical inactivation of viruses. Without any doubt, this approach is promising, but in the formation of complexes between the target and the P&A due to p-p-interaction, axial coordination, H-bonding, electrostatic interaction, there always remains the option of destruction (collapse) of the complexes. In addition, it should be borne in mind that for a positive antiviral effect, the required amount of P&A can be significant. Firstly, this follows from the basics of thermodynamics and is associated with the need for an excess of P&A to increase the number of the combined surface protein structures. Secondly, the number of spines themselves on the virion of viruses is very different: on the surface of the HIV-1 virion there are about 7-14 spines (Env) [9], influenza virus has 400-500 spines, vesicular stomatitis virus - about 1200, and the more of them, the greater the number of P&A required. Thirdly, the problem of target linking selectivity arises: part of the P&A can interact with other biosubstarts. In addition, the determination of the required dose of P&A should be correlated with the degree of damage to the body. And finally, another equally important factor – like resistance to bacterial antibiotics – is the resistance to antiviral drugs that also remains an urgent issue [10], because with chemical inhibition the virus still exists, it is only inactivated. In this sense, the use of photoinactivation of viral particles seems more promising, since photoinactivation involves the complete destruction of the viral infection.
It is well known that the P&As of porphyrin, chlorine, and phthalocyanine series are capable of absorbing light energy and generating reactive oxygen species. This process can proceed according to two mechanisms - type I and type II [11], the essence of which is as follows: in the ground state, the P&A is a singlet, the absorption of a photon of light leads to the excitation of one electron and its transition to an orbit with a higher energy.
The singlet state is short-lived (nanosecond lifetime), and excess energy can be emitted by the P&A in the form of light (fluorescence) or dissipated into the energy of disordered processes and, ultimately, into heat. The third option, ‘intersystem crossing’, is also possible, in which the P&A goes into a more stable excited triplet state (microsecond lifetime). Such a long from the point of view of photochemistry triplet state allows the P&A to transfer energy upon contact with molecular oxygen (O2); as a result, the P&A returns to its original singlet state, and oxygen passes into the excited triplet state (1O2). The described photochemical reaction is called the type II photochemical process [12]. A type I photochemical process can occur when an P&A photosensitizer in an excited state undergoes electron transfer reactions which ultimately lead to the formation of reactive oxygen species (ROS). Their formation occurs as follows: at the first stage, electron transfer is carried out with the formation of a cation/anion radical. A radical anion can react with oxygen to form a superoxide anion radical (O2•-). The dismutation or one-electron reduction of O2•- produces hydrogen peroxide (H2O2), which, in turn, can undergo another one-electron reduction with the formation of hydroxyl radicals (HO•) [11]. Active oxygen species obtained during photoexposure to the P&A will react with the immediate environment of the P&A and lead to irreversible chemical changes in the target. According to a number of researchers [11][13], the majority of P&As used in photoinduced oxidative processes of biomolecules "operate" via a type II mechanism. This opinion is based on a comparison of the rate constants of energy transfer processes in the production of 1O2 (k ~ 1–3 × 109 dm3 ∙ mol-1 ∙ s-1) and electron transfer in the production of O2•- (k ≤1 × 107 dm3 ∙ mol-1 ∙ s-1) [14]. According to other researchers, both mechanisms proceed simultaneously, and the contribution to the photooxidation of each of them depends on the nature of the P&A, the excitation wavelength [15], the duration and intensity of photoirradiation [16], and on other external conditions.
Thus, numerous studies have shown that P&A compounds can act as virucidal chemotherapeutic and photoinactivating compounds at the stage of attachment of the virion to the cell surface (receptor binding) and fusion with the cell membrane, regardless of the presence or absence of supercapsade. It is likely that the degree of virulence of the P&A will depend significantly on the localization of the P&A and the selectivity of linking with a target. For targeted modelling of the MGC structure reliable information on the structure of the target is needed. There is a definite correlation between virucidal activity and chemotherapeutic activity and photoinactivating ability. As a rule, P&As exhibiting both of the latter properties are virucidal [17][18].
3. Inactivation at the stage of transcription and replication of viral RNA/DNA
As noted above, HIV virion contains three enzymes: reverse transcriptase (RNA dependent DNA polymerase) which is involved in the synthesis of DNA on an RNA matrix; an integrase that catalyzes the incorporation of the generated viral DNA into the chromosome of the host cell; a protease that splits synthesized polyproteins into structural proteins. The stage of transcription and replication of viral RNA / DNA is impossible without the participation of these enzymes which are the target for chemotherapeutic agents used in the treatment of AIDS.
The significance of the nature of the metal complexing agent in the processes of HIV inactivation is evidenced by the results of [19]. According to them, reverse transcriptase is inhibited by porphyrins containing aminosulphonyl peripheral substituents. In this case, the metal-free porphyrin and its zinc complex were active at 33% and 6.4% inhibition, respectively, while the vanadium complex of porphyrin showed much better results (5 μM with an inhibition of more than 97%). However, as shown in [20], porphyrins demonstrated a low selectivity for reverse transferase binding. The selective binding of reverse transferase with iron-protoporphyrin dimethyl ether, protoporphyrin dimethyl ether and its sodium salt has been established [21], but the virucidal activity of natural porphyrins has not been evaluated.
Porphyrin compounds containing carborane esters as peripheral substituents have a greater inhibitory effect on the protease compared with protoporphyrin IX and porphyrins not containing carboranes. The introduction of metals, both coordinatively saturated and unsaturated, negatively affects the ability of the P&A to inhibit the protease. Important factors are not only the presence of carborane groups but also their isomerism. The introduction of a methyl group into carboran also significantly reduces the affinity of P&A linking with the protease. Apparently, carborane substituents specifically interact with HIV protease and it leads to a high affinity between the P&A and the enzyme. Replacing carborane cells with similar in size, but less hydrophobic groups, such as benzoyl, adamantoyl, b-napthoyl, allows one to obtain inhibitors in the low micromolar range, and it indicates the importance of hydrophobic interactions in stabilizing the complex of the P&A with the enzyme. The best result was obtained with porphyrin - tetrakiscarborane carboxylate ester of 2,4-bis-(α,b-dihydroxyethyl) deuteroporphyrin IX which is a submicromolar HIV protease inhibitor [22]. Moreover, for HIV-1 protease the IC50 value is 185 nM, while for HIV-2 it is 700 nM.
The stage of transcription and replication of viral RNA / DNA is naturally impossible without the source of genetic material. Viral genomes contain DNA or RNA which can be eitherdouble-stranded or single-stranded, linear or circular in topology and monocistronic or polycistronic. Genomes can be divided into several segments [23]. Reflecting the diversity of genetic material and replication mechanisms, Baltimore's classification divides viruses into seven groups: double-stranded (ds) DNA genomes (group I), single-stranded (ss)DNA genomes (group II), dsRNA genomes, (group III), ss(+)RNA genomes (group IV), ss(-)RNA genomes in (group V), ssRNA genomes with reverse transcription of the dsDNA replication of intermediate (group VI) and dsDNA genomes with the ssRNA replication intermediate (Group VII) [24]. The genome of the vast majority of viruses has been decoded, and can be found in specialized literature. Obviously, this genetic material of the virus is a supertarget for the fight against viral infection. Moreover, the linking of the P&A with the genetic material of the virus and its further photoinactivation are very promising.
Porphyrins and their analogues can form various types of complexes with DNA: intercalates (internal complex) [25], binding to a small groove of DNA [25], binding to a large groove of DNA [25] and external binding with self-stacking along the DNA surface (external complex) [25].
The way of linking porphyrins with DNA depends on the nature of the peripheral substituents of the P&A, on the metal of the complexing agent, the type of DNA [26][27][28], external conditions, and even the concentration of the P&A [25]. When changing the composition of the medium or the molar ratio R (R ratio is the ratio of the concentration of DNA base to porphyrin, [DNA] / [P] ranges), the internal complex can transform into the external [25][28][29]. As a rule, the formation of intercalation complexes requires the planar structure of the P&A, small peripheral substituents, the P&A:DNA ratio (for nitrogen base pairs) of less than 1:4. Cationic P&As are preferred since phosphate groups of DNA are negatively charged. The formation of intercalation of metal complexes of the P&A with DNA containing unsaturated or multiply charged metals is difficult or does not occur at all, due to steric hindrances caused by the existing axial ligand or metal counterion [25].
The thermodynamic stability of the complexes of P&A with DNA depends not only on the nature of P&A, but also on the type of complex formed [29]. This condition is important when using P&A as a chemotherapeutic agent, as well, as it will be shown below, when P&A is applied as a photoinactivator.
As our own [25] and literary studies [30][31][32] have shown, depending on the type of photochemical process I or II, different results of DNA photooxidation can be obtained. The process according to mechanism I leads to oxidation of bases, oxidation of a phosphoric ester group and, as a consequence, DNA cleavage [33]. The process according to mechanism II causes deamination, release of free purine bases, oxidation of a phosphoric ester group and leads to DNA fragmentation [25][33][34].
The result of photoirradiation of complexes of P&A with DNA substantially depends on the type of complex being formed. For example, in [25] it was shown that irradiation of porphyrin intercalates (TMPyP3 and TMPyP4) with DNA leads to DNA fragmentation, and in the case of TMPyP4, DNA fragments of different sizes are formed. Irradiation of external complexes of porphyrins with DNA results in the cleavage of DNA (TMPyP3 and TMPyP4) and is accompanied by photolysis of porphyrin (TMPyP3).
Thus, the formation of complexes between the P&A and DNA / RNA viruses can result in chemical and / or photochemical inactivation. Currently, the possibility of inactivation of viruses in vitro and in vivo is mainly determined by the mechanism of chemical inactivation. In this case, there is a potential danger, since the chemical modification of the genome may not inactivate the virus, but lead to a mutation [35]. The main difficulty in using P&A to inactivate viruses with a molecular target (the genetic material of viruses) lies in the low selectivity of P&A and the active binding of P&A to DNA by host cells.
Perhaps, the solution to this problem is not far off. A chemical approach to DNA recognition [36] based on the use of polyamides has already been found. Analogues of the N-methylpyrrole rings of these polyamides provide a set of five-membered heterocycles that can be combined (in the form of asymmetric pairs of rings) in a modular manner to recognize predetermined DNA sequences with affinity and specificity comparable to DNA-binding proteins. The presence of such a peripheral substituent in the P&A can solve the problem of precise targeting of viral DNA.
Another possible solution to the problem of selectivity for targeting the P&A to the viral genome is to accurately target the sections of the genetic material of viruses, such as, for example, G-quadruplexes. Guanine-rich RNA or DNA sequences are capable of folding and accepting four strands, called G-quadruplexes or "G4". Unique features of the G4 topology, very different from DNA duplex or single-stranded RNA, make it a potential therapeutic target. The orientation of the strands determines a parallel, antiparallel, or mixed topology of G4, and it is directly related to the conformational state, the anti- or syn-glycosidic bond between the base G and sugar [37].
4. Conclusion
In conclusion, we would like to note that the chemo- and photoinactivation of P&A viruses is very promising and can become an alternative and highly effective way to treat viral infections. To increase the selectivity of the action of P&A on viral targets, a targeted modification of the structure of P&A is necessary. The complexity and versatility of the objects of research emphasize the need to combine the efforts of chemists, microbiologists, virologists.
This entry is adapted from the peer-reviewed paper 10.3390/molecules25194368
References
- Cossart, P.; Helenius, A., Endocytosis of viruses and bacteria. Cold Spring Harbor perspectives in biology 2014, 6, (8), a016972.
- Delhalle, S.; Schmit, J.-C.; Chevigné, A., Phages and HIV-1: from display to interplay. International Journal of Molecular Sciences 2012, 13, (4), 4727-4794.
- Power, C., Lentiviruses and Macrophages: Molecular and Cellular Interactions. Expert Review of Anti-infective Therapy 2010, 8, (9), 985-986.
- Crispin, M.; Ward, A. B.; Wilson, I. A., Structure and immune recognition of the HIV glycan shield. Annual review of biophysics 2018, 47, 499-523.
- Vanyúr, R.; Héberger, K.; Jakus, J., Prediction of anti-HIV-1 activity of a series of tetrapyrrole molecules. Journal of chemical information and computer sciences 2003, 43, (6), 1829-1836.
- Gros, C. P.; Desbois, N.; Michelin, C. m.; Demilly, E.; Tilkin-Mariamé, A.-F. o.; Mariamé, B.; Gallardo, F., Synthesis and antiviral activity evaluation of nitroporphyrins and nitrocorroles as potential agents against human cytomegalovirus infection. ACS infectious diseases 2015, 1, (8), 350-356.
- Guo, H.; Pan, X.; Mao, R.; Zhang, X.; Wang, L.; Lu, X.; Chang, J.; Guo, J.-T.; Passic, S.; Krebs, F. C., Alkylated porphyrins have broad antiviral activity against hepadnaviruses, flaviviruses, filoviruses, and arenaviruses. Antimicrob. Agents Chemother. 2011, 55, (2), 478-486.
- Kensler, T. W.; Egner, P. A.; Wang, J.-B.; Zhu, Y.-R.; Zhang, B.-C.; Lu, P.-X.; Chen, J.-G.; Qian, G.-S.; Kuang, S.-Y.; Jackson, P. E., Chemoprevention of hepatocellular carcinoma in aflatoxin endemic areas. Gastroenterology 2004, 127, (5), S310-S318.
- Nanni, R. G.; Ding, J.; Jacobo-Molina, A.; Hughes, S. H.; Arnold, E., Review of HIV-1 reverse transcriptase three-dimensional structure: implications for drug design. Perspect. Drug Discovery Des. 1993, 1, (1), 129-150.
- Irwin, K. K.; Renzette, N.; Kowalik, T. F.; Jensen, J. D., Antiviral drug resistance as an adaptive process. Virus evolution 2016, 2, (1).
- Abrahamse, H.; Hamblin, M. R., New photosensitizers for photodynamic therapy. Biochem. J 2016, 473, (4), 347-364.
- Foote, C. S., Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science 1968, 162, (3857), 963-70.
- Bacellar, I. O.; Tsubone, T. M.; Pavani, C.; Baptista, M. S., Photodynamic efficiency: from molecular photochemistry to cell death. International journal of molecular sciences 2015, 16, (9), 20523-20559.
- Davies, M. J., Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, (3), 761-770.
- Lebedeva, N. S.; Yurina, E. S.; Gubarev, Y. A.; Lyubimtsev, A. V.; Syrbu, S. A., Effect of irradiation spectral range on porphyrin—Protein complexes. J. Photochem. Photobiol. A: Chem. 2018, 353, 299-305.
- Costa, L.; Carvalho, C. M.; Faustino, M. A.; Neves, M. G.; Tomé, J. P.; Tomé, A. C.; Cavaleiro, J. A.; Cunha, Â.; Almeida, A., Sewage bacteriophage inactivation by cationic porphyrins: influence of light parameters. Photochemical & Photobiological Sciences 2010, 9, (8), 1126-1133.
- Nikolaeva-Glomb, L.; Mukova, L.; Nikolova, N.; Kussovski, V.; Doumanova, L.; Mantareva, V.; Angeloc, I.; Wöhrle, D.; Galabov, A., Photodynamic effect of some phthalocyanines on enveloped and naked viruses. Acta Virol. 2017, 61, 341-346.
- Teles, A. V.; Oliveira, T. M.; Bezerra, F. C.; Alonso, L.; Alonso, A.; Borissevitch, I. E.; Gonçalves, P. J.; Souza, G. R., Photodynamic inactivation of bovine herpesvirus type 1 (Bohv-1) by porphyrins. J. Gen. Virol. 2018, 99, (9), 1301-1306.
- Wong, S.-Y.; Sun, R. W.-Y.; Chung, N. P.-Y.; Lin, C.-L.; Che, C.-M., Physiologically stable vanadium (IV) porphyrins as a new class of anti-HIV agents. Chem. Commun. 2005, (28), 3544-3546.
- Argyris, E. G.; Vanderkooi, J. M.; Venkateswaran, P.; Kay, B. K.; Paterson, Y., The connection domain is implicated in metalloporphyrin binding and inhibition of HIV reverse transcriptase. J. Biol. Chem. 1999, 274, (3), 1549-1556.
- Lin, L.; Hu, J., Inhibition of hepadnavirus reverse transcriptase-ε RNA interaction by porphyrin compounds. J. Virol. 2008, 82, (5), 2305-2312.
- DeCamp, D. L.; Babe, L. M.; Salto, R.; Lucich, J. L.; Koo, M. S.; Kahl, S. B.; Craik, C. S., Specific inhibition of HIV-1 protease by boronated porphyrins. J. Med. Chem. 1992, 35, (18), 3426-3428.
- Fermin, G., Virion Structure, Genome Organization, and Taxonomy of Viruses. Viruses 2018, 17.
- Aiewsakun, P.; Adriaenssens, E. M.; Lavigne, R.; Kropinski, A. M.; Simmonds, P., Evaluation of the genomic diversity of viruses infecting bacteria, archaea and eukaryotes using a common bioinformatic platform: steps towards a unified taxonomy. The Journal of general virology 2018, 99, (9), 1331.
- Lebedeva, N. S.; Yurina, E. S.; Gubarev, Y. A.; Syrbu, S. A., Interactions of tetracationic porphyrins with DNA and their effects on DNA cleavage. SPECTROCHIM ACTA A 2018, 199, 235-241.
- Banville, D. L.; Marzilli, L. G.; Strickland, J. A.; Wilson, W. D., Comparison of the effects of cationic porphyrins on DNA properties: influence of GC content of native and synthetic polymers. Biopolymers: Original Research on Biomolecules 1986, 25, (10), 1837-1858.
- Carvlin, M. J.; Fiel, R. J., Intercalative and nonintercalative binding of large cationic porphyrin ligands to calf thymus DNA. Nucleic Acids Res. 1983, 11, (17), 6121-6139.
- Lee, S.; Lee, Y.-A.; Lee, H. M.; Lee, J. Y.; Kim, D. H.; Kim, S. K., Rotation of Periphery Methylpyridine of meso-Tetrakis (n-N-methylpyridiniumyl) porphyrin (n= 2, 3, 4) and Its Selective Bindingto Native and Synthetic DNAs. Biophys. J. 2002, 83, (1), 371-381.
- Dutikova, Y. V.; Borisova, O.; Shchyolkina, A.; Lin, J.; Huang, S.; Shtil, A.; Kaluzhny, D., 5, 10, 15, 20-Tetra-(N-methyl-3-pyridyl) porphyrin destabilizes the antiparallel telomeric quadruplex d (TTAGGG) 4. Mol. Biol. 2010, 44, (5), 823-831.
- Da Ros, T.; Spalluto, G.; Boutorine, A. S.; Bensasson, R. V.; Prato, M., DNA-photocleavage agents. Curr. Pharm. Des. 2001, 7, (17), 1781-1821.
- Abe, H.; Ikebuchi, K.; Wagner, S.; Kuwabara, M.; Kamo, N.; Sekiguchi, S., Potential involvement of both Type I and Type II mechanisms in M13 virus inactivation by methylene blue photosensitization. Photochem. Photobiol. 1997, 66, (2), 204-208.
- Costa, L.; Faustino, M. A.; Tomé, J. P.; Neves, M. G.; Tomé, A. C.; Cavaleiro, J. A.; Cunha, Â.; Almeida, A., Involvement of type I and type II mechanisms on the photoinactivation of non-enveloped DNA and RNA bacteriophages. J. Photochem. Photobiol. B: Biol. 2013, 120, 10-16.
- Sobotta, L.; Skupin-Mrugalska, P.; Mielcarek, J.; Goslinski, T.; Balzarini, J., Photosensitizers mediated photodynamic inactivation against virus particles. Mini Reviews in Medicinal Chemistry 2015, 15, (6), 503-521.
- Wiehe, A.; O'Brien, J. M.; Senge, M. O., Trends and targets in antiviral phototherapy. Photochemical & Photobiological Sciences 2019, 18, (11), 2565-2612.
- Wainwright, M., Local treatment of viral disease using photodynamic therapy. Int. J. Antimicrob. Agents 2003, 21, (6), 510-520.
- Dervan, P. B.; Edelson, B. S., Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr. Opin. Struct. Biol. 2003, 13, (3), 284-299.
- Burge, S.; Parkinson, G. N.; Hazel, P.; Todd, A. K.; Neidle, S., Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 2006, 34, (19), 5402-5415.