The natural abundance of heavy stable isotopes (13C, 15N, 18O, etc.) is now of considerable importance in many research fields, including human physiology. In fact, it varies between tissues and metabolites due to isotope effects in biological processes, that is, isotope discriminations between heavy and light isotopic forms during enzyme or transporter activity.
- isotope effect
- fractionation
- metabolic partitioning
- diabetes
- cancer
- metal homeostasis
1. Introduction
In the second part of the XXth century, stable isotopes have then been exploited in medicine and biomedical research, mostly using enriched material (isotopic labelling with heavy water2H2O,13C-enriched glucose, leucine or urea, …) to quantify water turn-over, follow blood glucose homeostasis or trace the fate of precursors in metabolic pathways (for reviews, see [1][2][3]). Intense efforts are currently devoted to set up diagnostic procedures for metabolism-based pathologies using isotopic labelling. The use of isotopically enriched material has two drawbacks: first, it is rather expensive and second, feeding or injecting isotopic products may be associated with long procedures to address ethical or safety imperatives 536/2014), although the safety of using stable isotopes is well established [4][5].
During the past two decades, key advances have been made in our knowledge of natural isotope abundance (i.e., without isotopic enrichment) to take advantage of small but detectable differences in natural isotope content between patients and controls, associated with quite a range of pathologies. In fact, all of the natural elements are present in the form of various isotopic forms (e.g.,12C and13C for carbon) and some changes in isotope ratios (i.e., fractionations) have been found to be specific of diseases, reflecting key alterations in metabolism. In this entry, we summarize the current knowledge in fractionations associated with human diseases and discuss the potential of using isotopes at natural abundance for medical diagnosis and/or prognostic.
2. Basics of Stable Isotopes and Metabolic Isotope Effects
Elements forming biological tissues have different stable isotopes, like carbon (12C and13C) and nitrogen (14N and15N) for which the heavy form represents about 1.1 and 0.37%, respectively. These differences are mostly due to isotope effects, whereby the velocity of enzymatic reactions or transport phenomenon differ between isotopic forms (in theAppendix A,Box A1for definitions). The isotope discrimination (or fractionation), denoted as Δ, is often quantified using the difference between substrate and product isotope composition (or delta value, denoted as δ13C, δ15N, etc.), i.e., Δ = δsubstrate− δproduct. The term “isotope composition” refers to the isotope ratio relative to the international standard (usually measured with isotope ratio mass spectrometry, in theAppendix A,Box A2).
The fact that isotope effects arise from enzyme action or transport explains why changes in metabolic pathways often lead to changes in delta values. Alterations in delta values can also stem from a source effect, whereby the origin and thus the delta value of the substrate has changed. The bladder stone has been analysed layer after layer, and the δ13C value has been found to correlate positively to calcium oxalate content while the δ15N value correlated negatively to struvite (magnesium ammonium phosphate, MgNH4PO4∙6 H2O) content [6]. This indicates that (i) the δ13C of excreted oxalate was very high (near −10‰) pointing to a plant origin (oxalic-rich food such as strong black tea and low-value vegetables), and (ii) the δ15N of ammonia was relatively depleted (near +4‰) reflecting the isotope effect in amino acid deamination reactions.
Medical applications of natural abundance to detect pathologies are based on these principles, that is, a change in delta values caused by alterations in metabolism, nutritional conditions, or recycling efficiency (e.g., hepatic remobilisation). There has been an exponential increase in studies looking at potential changes in delta values in hair, blood (serum or clot), or other sample types associated with a large range of diseases, from metabolic disorders to cancer (summarized in Table 1). This implies that it is preferable to use relative (i.e., isotopic offset with respect to food intake delta values) rather than absolute delta values. Second, cohorts must be formed with care since there can be unforeseen isotopic differences caused by common medical treatments, local nutritional habits and also importantly, sex [7].
Table 1. Summary of documented examples of pathologies where isotopes at natural abundance could be used for potential diagnostics. Aa, amino acid; ND, not determined. The term “metabolic mechanism” refers to the major pathways explaining the change in isotope abundance.
| Disease | Metabolic Mechanism | Isotopic Marker | Matrix | Ref. |
|---|---|---|---|---|
| Nervous anorexia, nutritional stress | Aa metabolism | 13C, 15N | Hair | [8][9] |
| Syphilis | Aa metabolism | 13C, 15N | Collagen | [10] |
| Chronic malnutrition and potential growth retardation (stunted children) | Aa metabolism | 13C, 15N | Hair | [11] |
| Patients with metabolic syndrome | Glycaemia Aa metabolism | 13C, 15N | Hair | [12] |
| Diabetic patients | Sugar metabolism | 13C, 15N | Hair | [13][14][15][16] |
| Cirrhotic patients | Aa metabolism | 13C, 15N | Hair, bulk protein | [17] |
| Breast cancer | Urea cycle, glycolysis, lipid synthesis, anaplerosis | 13C, 15N | Tissue biopsies cultured cells | [18][19] |
| Oral squamous cell carcinomas | ND | 13C, 15N | Tissue biopsies | [20] |
| Ganglioneuroma (benign tumours), neuroblastoma and nephroblastoma Wilm’s tumours | Aa metabolism | 13C, 15N | Tissue biopsies | [21][22] |
| Rhabdomyosarcoma | ND | 13C, 15N | Tissue biopsies | [23] |
| Adrenal gland cancers | Aa metabolism Glycolysis | 13C, 15N | Serum | Unpublished data |
| Hepatocarcinoma | Glutathione metabolism, | 34S | Serum and erythrocytes | [24] |
| Wilson disease | Cu metabolism | 65Cu | Serum | [25] |
| Menkes disease | Cu and Aa metabolism | 15N | Hair | Unpublished data |
| Ovarian cancer | Cu metabolism | 65Cu | Serum | [26] |
| Homeostasis alterations after bariatric surgery | Zn homeostasis | 66Zn | Serum and Whole blood | [27] |
| Hematological malignancy | Metal homeostasis | 65Cu, 66Zn | serum | [28] |
| Anaemia | Fe deficiency | 56Fe | Whole blood | [25] |
| Multiple myeloma | Bone formation (apatite deposition) | 44Ca | Serum and urine | [29] |
| Chronic kidney disease or diabetes | Bone formation (apatite deposition) | 44Ca | Serum | [30] |
| Anaemia in skeleton fragments | Respiratory biochemistry | 18O | Bone and enamel apatite | [31] |
| Osteopenia and osteoporosis in female skeleton | Urea excretion and/or renal function | 15N | Bone collagen | [32] |
| Cealiac disease in skeleton | Aa metabolism | 13C, 15N | Bone collagen | [33] |
There is now considerable evidence that pathologies directly related to metabolism (nutritional stress, malnutrition, metabolic syndrome in general, diabetes, and obesity) have an impact on natural isotope abundance. Pre-clinical studies with rats subjected to caloric restriction, normal or high fat diet regimes have demonstrated that caloric restriction causes a general decline in peripheric protein content but the impact on isotope compositions varies between organs [34]. Such variations are due to changes in amino acid homeostasis, whereby liver oxidises more amino acids and this process discriminates between isotopes (against15N). Therefore, amino acids left behind and available for protein synthesis are15N-enriched.
In humans, several studies took advantage of the delta value of easily accessible samples (blood, hairs) in prediabetic patients or in association with physiological variables. A typical situation has been found in patients affected by nervosa anorexia and nutritional stress during pregnancy, whereby the δ15N values in hair increases, showing the involvement of recycling leading to15N-enriched amino acids [8][9]. The relationship between isotope abundance in hair and nutritional stress has been reviewed elsewhere [35]. Similarly, in children from Bangladesh with chronic malnutrition and potential growth retardation (stunted children), hairs are both13C- and15N-depleted [11].
However, quantitative relationships (regressions) are only significant with glycaemic index and waist circumference. In a comparison of diabetic patients with controls, no change in hair δ15N was found while the δ13C value declined and a relationship was found with haemoglobin A1 glycation (HbA1c), this relationship being mostly visible in males [13]. However, in another cohort, hair δ15N correlated to plasmatic leptin concentration (in particular in individuals with high body mass index) but no relationship was found with δ13C values [14]. In adolescents or children, the δ13C value in fingerstick blood samples or in erythrocytes has been found to increase at high food intake (increased sugar intake or high calory diet) and this seemed to be unrelated to HbA1c or to C4sugar consumption [15][36].
Since amino acid metabolism (via transamination) is very active in the liver and is associated with different isotope effects [37], changes in δ15N values (and potentially, δ13C values as well) can be anticipated in metabolic diseases affecting liver function, including in cirrhotic patients. [17] compared the δ15N and δ13C values of hair from patients with liver disease to healthy controls. Bulk protein δ15N was 3.2‰ lower in cirrhotic patients compared to controls without any significant differences in δ13C. Additionally, nearly all amino acids had a lower δ15N in cirrhotic individuals.
Such physio-pathological factors of obesity and metabolic syndrome, which vary between patients, probably contribute to isotopic variations. Noteworthy, isotopic variations also likely come from differences in country-specific food intake behaviour and thus how patients with diabetes and metabolic syndrome readjust their diet. It has been suggested that (pre)diabetic patients have an altered carbonic anhydrase activity, causing a change in CO2-water oxygen equilibration in tissues and thus a specific δ18O value in exhaled CO2[16]. In effect, δ13C values of exhaled breath CO2in ventilated paediatric patients (infants) in intensive care unit were not different between groups with systemic inflammatory response syndrome (SRIS), no SIRS and SIRS with shock; however, breath δ13C value was significantly lower in patients with active sepsis (septic shock), trauma, or after surgery compared to other individuals.
3. Isotope Fractionation in Cancer
Over the past two decades, isotopes of both macro-elements (C, N, S) and metals have been investigated in biological samples from patients with cancer. In this section, we shall focus on macro-elements so as to relate to potential alterations in cancer metabolism. Metal stable isotopes in cancer are addressed in the next section.
Presumably, metabolism deregulation in cancer should lead to strong alterations in the abundances of natural isotopes13C,15N and34S since many metabolic pathways accompany oncogenesis. In particular, cancer cells adapt their metabolism to maximize the use of N and C sources for anabolism and biosynthesis of macromolecules required by cell proliferation and tumour growth [38]. How delta values vary in cancerous cells and tissues was unknown until the first investigation was released in a patent based on EA-IRMS technology (seeAppendix A,Box A2) applied to biological fluids or tissues associated with cancer [19]. In what follows, we start with breast cancer (BrCa), which is presently the best documented cancer type as isotopes are concerned.
δ13C and δ15N values have been measured on a set of both exeresis samples from patients and cultured BrCa cell lines, showing that cancerous cells with propensity to be invasive Furthermore, by using compound-specific analyses (seeBox A2), it has been demonstrated that the generation of15N-depleted arginine and urea by the UC is likely to be at the origin of the15N depletion in cancerous cells. via carbamoyl phosphate and thus, the arginine build-up contributes to the natural13C enrichment in cancerous cells (summarized in Figure 1A). It is worth noting that delta values have a good potential to distinguishing BrCa subtypes, since they have been shown to have different metabolic phenotypes [39].
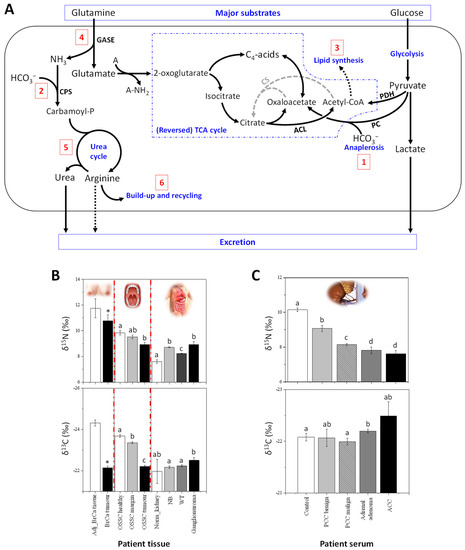
Other cancers also appear to be associated with alterations in delta values consistent with BrCa. Oral tissue δ15N has been shown to decrease in patients with oral squamous cell carcinomas (OSCC), while δ13C values increase [20]. Also, δ15N and δ13C values slightly differ between tissues taken from margin and distant oral mucosa (Figure 1B). Interestingly, this study also revealed that tumour δ13C is positively correlated with alcohol consumption and occurrence of angioinvasion, and inversely related with BMI index.
In infants or children, the δ15N values in tissues from ganglioneuroma (benign tumours), neuroblastoma and nephroblastoma (Wilm’s tumours, WT, which are malignant) have been found to be higher compared to normal kidney cortex tissue, used as a control [21][22] (summarized in Figure 1B). Isotopic signatures of WT in subsequent stages of cancer disease have also been studied and WT tissues were15N-depleted and13C-enriched in stage 3 of the disease compared to stage 2. In this context, low δ15N values might be good biomarkers of the worst prognosis [22]. Five-year survival is observed in approximately 82% for ERMS and 65% for ARMS [23], and the prognosis for patients with progressive disease is still poor.
In adults,15N-depletion in serum was also found in different types of adrenal gland cancers (Figure 1C) compared to healthy patients (unpublished data from our laboratory). The serum of patients with contrasting types of adrenal tumors (i.e., ACC, adrenal adenoma and PPC, pheochromocytoma) has been analysed and low δ15N values significantly correlate to malignancy (Figure 1C). For example, patients with ACC have a naturally15N-depleted serum compared to patient with PCC or adrenal adenomas. Moreover, in the group of patients with PCC, patients with benign tumours can be differentiated from those with malignant tumours on a δ15N basis.
This suggests a strong impact of malignancy on PCC cancer cell metabolism, which in turn affects δ15N values in circulating metabolites. Although the metabolism of the different types of adrenal gland cancer is not well-known, an interesting feature is that the glucose transporter (GLUT1) is a promising prognostic marker of ACC [40]. Since glucose entry and insulin-based regulation is essential for protein turn-over signalling and amino acid cellular homeostasis, it is possible that changes in δ15N stem from an imbalance between protein synthesis and degradation within adrenal cancer cells. We also recognize that a general effect on protein metabolism and thus on serum δ15N due to cancer development is possible, in addition to a tumour-specific effect.
The first δ34S values in patients with cancer were obtained using EA-IRMS analysis, and it has been found that both serum and erythrocytes are significantly34S-depleted in patients with hepatocellular carcinoma (HCC) compared to controls [24]. In addition, glutathione (GSH) metabolism which plays important roles in cancer cells [41] could be involved, via oxidative stress response and the redox balance between extracellular cysteine and cysteine. In that context, the34S-depletion in the serum of patients with HCC could come from a more oxidised status. It is important to note δ34S values in serum of patients with BrCa or prostate cancer are not different from that in controls [42], showing that δ34S are probably specific to alterations in liver metabolism.
Presently, despite the rather small number of studies, it is clear that δ15N, δ13C and δ34S values have the potential to correlate to cancer development. Of course, since this effect is mostly caused by specific features of cancer cell metabolism, further studies with compound-specific analyses are required. not only to better understand the metabolic origin of isotope signatures of cancer but also to find a reliable isotopic biomarker of cancer that is independent of nutrition and therefore that do not require control samples for systematic comparison.
4. Isotope Fractionation in Metal Homeostasis
Isotope fractionations associated with four essential metals (Fe, Cu, Zn and Ca) as well as isotope compositions in various biological samples, including plants, animals and humans have been extensively reviewed in recent publications [25][43][44]. Here, we will very briefly explain principles of metal homeostasis and present latest studies dealing with metal isotope fractionation in human pathologies.
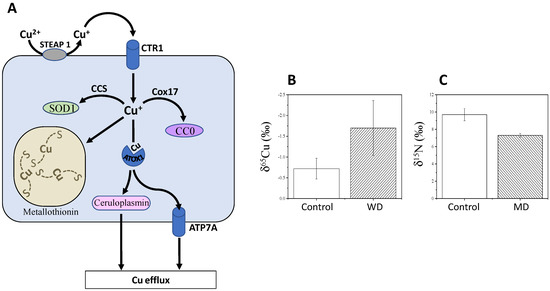
In principle, variations in65Cu/63Cu ratios are due to the change in oxidation state (i.e., from Cu2+to δ65Cu values in cells are lower than that in the diet, because Cu entering the cell is in its reduced form, which is65Cu-depleted (isotope effect in reduction). Cu is then transported from the intestine to the liver and used to synthesise Cu-containing proteins, such as copper chaperone for superoxide dismutase (CCS, which delivers Cu to superoxide dismutase, SOD1), cytochrome c copper chaperone (Cox17) (which delivers copper to cytochrome c oxidase, CCO), ATP7A/7B (copper transporters) and ceruloplasmin (major copper-carrying protein in blood). In addition to the change in oxidation state, forming specific chemical bonds also fractionates between Cu isotopes.
Because of its involvement in SOD1 catalysis, Cu is involved in the mitigation of reactive oxygen species (ROS), which are thus influenced by intestinal absorption and bile excretion of Cu [25]. Alterations in Cu homeostasis can cause serious diseases, such as Wilson and Menkes diseases [25]. The Menkes disease (MD) involves a mutation in ATP7A and leads to copper deficiency and thereby strong neurodegenerative disorders. Low δ65Cu values are observed in the serum of patients with WD, compared to healthy subjects (Figure 2C).
Lamboux and co-workers [45] reported recently that healthy subjects and naïve (non-treated) patients with WD had undistinguishable δ65Cu, and treated patients had the same δ65Cu values regardless of treatment type and duration. However, the variation in serum δ65Cu was negatively correlated with the degree of liver fibrosis. This suggests that the inability for Cu recirculation from the liver is at the origin of the65Cu-depletion in general circulation, regardless of the genetic background and treatment. These results suggest that δ65Cu is not a good biomarker of ATP7B mutation but rather, has some potential as a prognostic biomarker for evaluating the progression of liver fibrosis in WD.
Interestingly, changes in δ65Cu can also be observed in diseases other than WD and MD. For example, in patients with ovarian cancer, δ65Cu values in plasma are lower than in healthy controls [26]. δ65Cu values in ovary tumour tissues are higher than in adjacent healthy tissues [26], simply suggesting a mass-balance effect whereby the increased Cu influx in tumours forms65Cu-enriched copper lactate [24] and depletes healthy ovary tissues and blood in65Cu.
Recent studies used the combination of Cu, Fe and Zn isotopes in blood to assess possible homeostasis alterations after bariatric surgery and during the follow-up of haematological malignancy (HM) in patients [27][28].
Both serum and whole blood had lower δ65Cu values after bariatric surgery, reaching statistical significance at 6 months post-surgery [28]. By contrast, serum66Zn was slightly higher 6 months post-surgery than pre-surgery, but the difference did not reach statistical significance and furthermore, this enrichment in66Zn was not observed in whole blood. Still, the difference in δ66Zn value between serum and whole blood (expressed as Δ66Zn) became gradually larger over post-operative time. Presumably, the change in Δ66Zn might reflect a disruption in Zn homeostasis, Zn status or Zn absorption (and competition with Cu absorption) in patients with bariatric surgery.
Patients suffering from severe diabetes and Fe deficiency anaemia show high δ56Fe values in whole blood. Reciprocally, the Fe3+ion of transferrin (Tf) is reduced to Fe2+in haemoglobin and myoglobin, leading to a low δ56Fe value in red blood cells and muscles [25][43]. Therefore, metabolic isotope fractionations with Zn isotopes are numerically smaller than those found with Cu and Fe. They are attributable to the high binding energy of Zn with ligand molecules and thus effects of Zn adsorption and coordination at the root surface in plants, which are very different to biochemistry of animal Zn absorption.
In fact, MM is characterized by osteolytic lesions (bone mass loss) due to a relative increase in osteoclastic activity, liberating Ca2+and causing bone resorption. Primary urine has thus a higher load in Ca with relatively low δ44Ca (compared to normal primary urine), which is then reabsorbed into blood, explaining a low δ44Ca in serum of patients with MM [29]. [30] reported that serum and bone from rats with chronic kidney disease or diabetes had lower δ44Ca values than in controls. δ44Ca values were correlated to bone mineral density, suggesting a link with bone resorption and formation like in MM.
This entry is adapted from the peer-reviewed paper 10.3390/metabo11060370
References
- Huidekoper, H.H.; Wijburg, F.A.; Wanders, R. Inborn Errors of Metabolism. In Mass Spectrometry and Stable Isotopes in Nutritional and Pediatric Research; Schierbeek, H., Ed.; John Wiley & Sons: New York, NY, USA, 2017; pp. 258–283.
- Bodamer, O.A.F.; Halliday, D. Uses of stable isotopes in clinical diagnosis and research in the paediatric population. Arch. Dis. Child. 2001, 84, 444.
- Charidemou, E.; Ashmore, T.; Griffin, J.L. The use of stable isotopes in the study of human pathophysiology. Int. J. Biochem. Cell Biol. 2017, 93, 102–109.
- Jones, P.J.H.; Leatherdale, S.T. Stable isotopes in clinical research: Safety reaffirmed. Clin. Sci. 1991, 80, 277–280.
- Davies, P.S.W. Stable isotopes: Their use and safety in human nutrition studies. Eur. J. Clin. Nutr. 2020, 74, 362–365.
- Wang, L.; Chen, M.; He, P.; Yu, H.; Block, K.A.; Xie, Z. Composition and spatial distribution of elements and isotopes of a giant human bladder stone and environmental implications. Sci. Total Environ. 2019, 650, 835–846.
- Kraft, R.A.; Jahren, A.H.; Saudek, C.D. Clinical-scale investigation of stable isotopes in human blood: δ13C and δ15N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun. Mass Spectrom. 2008, 22, 3683–3692.
- Mekota, A.M.; Grupe, G.; Ufer, S.; Cuntz, U. Serial analysis of stable nitrogen and carbon isotopes in hair: Monitoring starvation and recovery phases of patients suffering from anorexia nervosa. Rapid Commun. Mass Spectrom. 2006, 20, 1604–1610.
- Fuller, B.T.; Fuller, J.L.; Sage, N.E.; Harris, D.A.; O’Connell, T.C.; Hedges, R.E.M. Nitrogen balance and δ15N: Why you’re not what you eat during nutritional stress. Rapid Commun. Mass Spectrom. 2005, 19, 2497–2506.
- Salesse, K.; Kaupová, S.; Brůžek, J.; Kuželka, V.; Velemínský, P. An isotopic case study of individuals with syphilis from the pathological-anatomical reference collection of the national museum in Prague (Czech Republic, 19th century A.D.). Int. J. Paleopathol. 2019, 25, 46–55.
- Dailey-Chwalibóg, T.; Huneau, J.F.; Mathé, V.; Kolsteren, P.; Mariotti, F.; Mostak, M.R.; Alim, M.A.; Khan, M.M.S.T.; Khan, M.A.H.; Guesdon, B.; et al. Weaning and stunting affect nitrogen and carbon stable isotope natural abundances in the hair of young children. Sci. Rep. 2020, 10, 2522.
- Park, J.-K.; Ahn, S.V.; Kim, M.K.; Lee, K.-S.; Koh, S.-B.; Bong, Y.-S. The association between carbon and nitrogen stable isotope ratios of human hair and metabolic syndrome. Clin. Chim. Acta 2015, 450, 72–77.
- Hotta, Y.; Fujino, R.; Kimura, O.; Fujii, Y.; Haraguchi, K.; Endo, T. Assessment of diabetics by the quantification of essential elements and stable isotope ratios of carbon and nitrogen in scalp hair. Obes. Med. 2019, 15, 100106.
- Ahn, S.V.; Koh, S.-B.; Lee, K.-S.; Bong, Y.-S.; Park, J.-K. Association between nitrogen stable isotope ratios in human hair and serum levels of leptin. Tohoku J. Exp. Med. 2017, 243, 133–139.
- Henze, M.M.; Bemis, E.A.; Seifert, J.A.; Johnson, R.K.; Dong, F.; Rewers, M.; Norris, J.M. Association between change in self-reported sugar intake and a sugar biomarker (δ13C) in children at increased risk for type 1 diabetes. J. Nutr. Sci. 2020, 9, e16.
- Ghosh, C.; Mandal, S.; Pal, M.; Mukhopadhyay, P.; Ghosh, S.; Pradhan, M. 13C isotopic abundances in natural nutrients: A newly formulated test meal for non-invasive diagnosis of type 2 diabetes. J. Breath Res. 2017, 11, 026005.
- Petzke, K.J.; Feist, T.; Fleig, W.E.; Metges, C.C. Nitrogen isotopic composition in hair protein is different in liver cirrhotic patients. Rapid Commun Mass Spectrom 2006, 20, 2973–2978.
- Tea, I.; Martineau, E.; Antheaume, I.; Lalande, J.; Mauve, C.; Gilard, F.; Barillé-Nion, S.; Blackburn, A.C.; Tcherkez, G. 13C and 15N natural isotope abundance reflects breast cancer cell metabolism. Sci. Rep. 2016, 6.
- Tea, I.M.E.; Giraudeau, P.; Akoka, S.; Barillé-Nion, S. Method to Characterize the Origin and/or the State of Pathological or Healthy cells, and Its Applications in Biology. E.P. Patent No. 2686686B1, 14 October 2015. Available online: (accessed on 7 May 2021).
- Bogusiak, K.; Puch, A.; Mostowski, R.; Kozakiewicz, M.; Paneth, P.; Kobos, J. Characteristic of Oral Squamous Cell Carcinoma Tissues Using Isotope Ratio Mass Spectrometry. J. Clin. Med. 2020, 9, 3760.
- Taran, K.; Frączek, T.; Kamiński, R.; Sitkiewicz, A.; Kobos, J.; Paneth, P. The first protocol of stable isotope ratio assessment in tumor tissues based on original research. Pol. J. Pathol. 2015, 66, 288–295.
- Taran, K.; Frączek, T.; Sikora-Szubert, A.; Sitkiewicz, A.; Młynarski, W.; Kobos, J.; Paneth, P. The first investigation of Wilms’ tumour atomic structure-nitrogen and carbon isotopic composition as a novel biomarker for the most individual approach in cancer disease. Oncotarget 2016, 7, 76726.
- Taran, K.; Frączek, T.; Sitkiewicz, A.; Paneth, P.; Kobos, J. Rhabdomyosarcoma in children in the light of isotope ratio mass spectrometry. Pol. J. Pathol. 2015, 66, 383–388.
- Balter, V.; Nogueira da Costa, A.; Bondanese, V.P.; Jaouen, K.; Lamboux, A.; Sangrajrang, S.; Vincent, N.; Fourel, F.; Télouk, P.; Gigou, M.; et al. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, 982–985.
- Tanaka, Y.K.; Hirata, T. Stable Isotope Composition of Metal Elements in Biological Samples as Tracers for Element Metabolism. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2018, 34, 645–655.
- Toubhans, B.; Gourlan, A.T.; Telouk, P.; Lutchman-Singh, K.; Francis, L.W.; Conlan, R.S.; Margarit, L.; Gonzalez, D.; Charlet, L. Cu isotope ratios are meaningful in ovarian cancer diagnosis. J. Trace Elem. Med. Biol. 2020, 62, 126611.
- Hastuti, A.; Costas-Rodríguez, M.; Anoshkina, Y.; Parnall, T.; Madura, J.A., 2nd; Vanhaecke, F. High-precision isotopic analysis of serum and whole blood Cu, Fe and Zn to assess possible homeostasis alterations due to bariatric surgery. Anal. Bioanal. Chem. 2020, 412, 727–738.
- Hastuti, A.A.M.B.; Costas-Rodríguez, M.; Matsunaga, A.; Ichinose, T.; Hagiwara, S.; Shimura, M.; Vanhaecke, F. Cu and Zn isotope ratio variations in plasma for survival prediction in hematological malignancy cases. Sci. Rep. 2020, 10, 16389.
- Gordon, G.W.; Monge, J.; Channon, M.B.; Wu, Q.; Skulan, J.L.; Anbar, A.D.; Fonseca, R. Predicting multiple myeloma disease activity by analyzing natural calcium isotopic composition. Leukemia 2014, 28, 2112–2115.
- Tanaka, Y.-k.; Yajima, N.; Higuchi, Y.; Yamato, H.; Hirata, T. Calcium isotope signature: New proxy for net change in bone volume for chronic kidney disease and diabetic rats. Metallomics 2017, 9, 1745–1755.
- Carroll, G.M.A.; Inskip, S.; Waters-Rist, A. Pathophysiological Stable Isotope Fractionation: Assessing the Impact of Anemia on Enamel Apatite d18O and d13C Values and Bone Collagen d15N and d13C Values. Bioarchaeology Int. 2018, 2, 117–146.
- Reitsema, L.J. Beyond diet reconstruction: Stable isotope applications to human physiology, health, and nutrition. Am. J. Hum. Biol. 2013, 25, 445–456.
- Scorrano, G.; Brilli, M.; Martínez-Labarga, C.; Giustini, F.; Pacciani, E.; Chilleri, F.; Scaldaferri, F.; Gasbarrini, A.; Gasbarrini, G.; Rickards, O. Palaeodiet reconstruction in a woman with probable celiac disease: A stable isotope analysis of bone remains from the archaeological site of Cosa (Italy). Am. J. Phys. Anthropol. 2014, 154, 349–356.
- Bernardo, K.; Jousse, C.; Fafournoux, P.; Schiphorst, A.M.; Grand, M.; Robins, R.J.; Hankard, R.; De Luca, A. Protein restricted diet during gestation and/or lactation in mice affects 15N natural isotopic abundance of organs in the offspring: Effect of diet 15N content and growth. PLoS ONE 2018, 13, e0205271.
- Petzke, K.J.; Fuller, B.T.; Metges, C.C. Advances in natural stable isotope ratio analysis of human hair to determine nutritional and metabolic status. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 532–540.
- Liu, S.V.; Moore, L.B.; Halliday, T.M.; Jahren, A.H.; Savla, J.; Hedrick, V.E.; Marinik, E.L.; Davy, B.M. Short-term changes in added sugar consumption by adolescents reflected in the carbon isotope ratio of fingerstick blood. Nutr. Health 2018, 24, 251–259.
- Sick, H.; Roos, N.; Saggau, E.; Haas, K.; Meyn, V.; Walch, B.; Trugo, N. Amino acid utilization and isotope discrimination of amino nitrogen in nitrogen metabolism of rat liver in vivo. Z. Fur Ernahr. 1997, 36, 340–346.
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer 2018, 18, 634–645.
- Lloyd, S.M.; Arnold, J.; Sreekumar, A. Metabolomic profiling of hormone-dependent cancers: A bird’s eye view. Trends Endocrinol. Metab. TEM 2015, 26, 477–485.
- Pinheiro, C.; Granja, S.; Longatto-Filho, A.; Faria, A.M.; Fragoso, M.C.; Lovisolo, S.M.; Lerário, A.M.; Almeida, M.Q.; Baltazar, F.; Zerbini, M.C. Metabolic reprogramming: A new relevant pathway in adult adrenocortical tumors. Oncotarget 2015, 6, 44403–44421.
- Lo, M.; Wang, Y.Z.; Gout, P.W. The x(c)- cystine/glutamate antiporter: A potential target for therapy of cancer and other diseases. J. Cell. Physiol. 2008, 215, 593–602.
- Albalat, E.; Telouk, P.; Balter, V.; Fujii, T.; Bondanese, V.P.; Plissonnier, M.-L.; Vlaeminck-Guillem, V.; Baccheta, J.; Thiam, N.; Miossec, P.; et al. Sulfur isotope analysis by MC-ICP-MS and application to small medical samples. J. Anal. At. Spectrom. 2016, 31, 1002–1011.
- Albarède, F.; Télouk, P.; Balter, V. Medical Applications of Isotope Metallomics. Rev. Mineral. Geochem. 2017, 82, 851–885.
- Albarede, F.; Télouk, P.; Balter, V.; Bondanese, V.P.; Albalat, E.; Oger, P.; Bonaventura, P.; Miossec, P.; Fujii, T. Medical applications of Cu, Zn, and S isotope effects. Metallomics 2016, 8, 1056–1070.
- Lamboux, A.; Couchonnal-Bedoya, E.; Guillaud, O.; Laurencin, C.; Lion-François, L.; Belmalih, A.; Mintz, E.; Brun, V.; Bost, M.; Lachaux, A.; et al. The blood copper isotopic composition is a prognostic indicator of the hepatic injury in Wilson disease. Metallomics 2020, 12, 1781–1790.
