Regular exercise is associated with pronounced health benefits. The molecular processes involved in physiological adaptations to exercise are best understood in skeletal muscle. Enhanced mitochondrial functions in muscle are central to exercise-induced adaptations. However, regular exercise also benefits the brain and is a major protective factor against neurodegenerative diseases, such as the most common age-related form of dementia, Alzheimer’s disease, or the most common neurodegenerative motor disorder, Parkinson’s disease.
- muscle
- exercise
- brain
- mitochondria
- myokines
- neurodegeneration
- neuroprtection
- Alzheimer disease
1. Introduction
The umbrella term “neurodegenerative disease” refers to the progressive pathological loss of neurons in specific neuronal circuits. Examples are Huntington’s disease, amyotrophic lateral sclerosis, spinocerebellar ataxia, and Alzheimer’s (AD) and Parkinson’s diseases (PD).
Currently no treatment strategies that modify the disease course of AD or PD exist, and symptomatic treatments are limited, in particular for AD. Lifestyle factors, notably including physical activity and exercise, however, are major factors in modulating the risk of developing these neurodegenerative diseases [5,6,7]. Exercise is defined as planned, structured, and repetitive physical activity that is performed with the objective to improve or maintain physical fitness [8]. An important aspect of both general physical activity and exercise is the activation and training of skeletal muscles, which is associated with enhanced mitochondrial function. Regular endurance exercise improves cardiorespiratory fitness and skeletal muscle function and has well-documented health effects, including reduced all-cause mortality [9]. It furthermore importantly influences mitochondrial health [10,11].
2. Mitochondrial Dysfunction in Neurodegenerative Diseases
2.1. Mitochondrial Respiration and Energy Provision
The brain has high energy requirements for which it mostly relies on oxidative energy metabolism [21]. It therefore critically depends on continuous adequate oxygen and substrate supplies. Mitochondrial oxidative phosphorylation (OXPHOS) is the most important energy-providing process in the brain. OXPHOS consists of the electron transport system (mitochondrial complexes I–IV) that establishes a proton gradient across the inner mitochondrial membrane. The resulting mitochondrial membrane potential is used by the second OXPHOS component, the phosphorylation system, to generate and transport ATP. Dysfunction of distinct complexes of the respiratory chain traditionally have been linked to different neurodegenerative diseases. Thus, for example complex I dysfunction has been attributed a prominent role in PD pathogenesis [12], complex IV in AD [12], and complex II in Huntington’s disease [22,23].
2.2. Mitochondrial ROS and Oxidative Stress
Supraphysiological high mitochondrial ROS levels result in the oxidative damage of multiple biomolecules, such as DNA, lipids, and proteins. This impairs cellular processes, such as the maintenance of membrane potential, trans-membrane transport, proteostasis, and enzyme activities, and is also implicated in the pathogenesis of many diseases [30,31]. Oxidative stress is a core mechanism in neurodegenerative disease pathogenesis [13]. This is reflected, for example, by increased levels of oxidative modifications in the substantia nigra of PD patients [32] and oxidative stress as an early pathological process in AD [33].
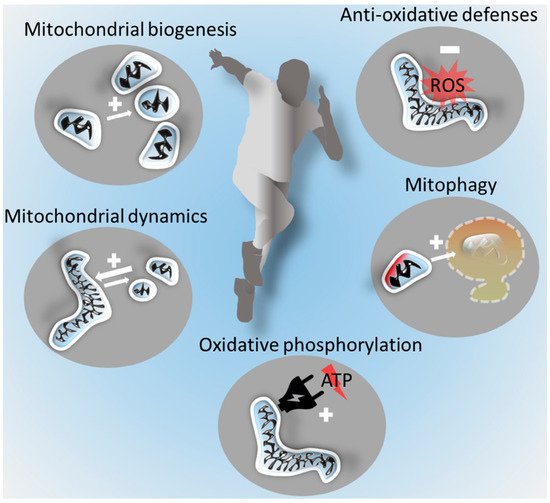
2.3. Mitochondrial Biogenesis
The increase of mitochondrial mass from pre-existing mitochondria (mitochondrial biogenesis) is controlled by numerous pathways and revolves around the cotranscriptional factor peroxisome-proliferator-activated receptor γ coactivator-1α (PGC-1α) and other molecules [27,34]. The PGC-1α-mediated activation of mitochondrial transcription factor A induces mitochondrial growth and division to generate new mitochondria [35]. An important regulator of mitochondrial biogenesis is the exercise-inducible PGC-1α activator AMP-activated protein kinase (AMPK) [35].
2.4. Mitochondrial Dynamics and Mitophagy
Mitochondria are highly dynamic organelles, which can change their number and size by fusion and fission [41]. Fusion denotes the process of several smaller separated mitochondria merging into one bigger mitochondrion, while fission refers to the division of one mitochondrion into several smaller mitochondria. In contrast to mitochondrial biogenesis and mitophagy, these processes are not associated with a change in mitochondrial mass but also influence mitochondrial function. Fusion of the outer mitochondrial membrane is effectuated primarily by mitofusin 1 (Mfn1) and Mfn2 and fusion of the inner mitochondrial membrane by optic atrophy 1 (Opa1). Mitochondrial fission is executed by dynamin-related protein 1 (Drp1), a cytosolic protein that is recruited to the mitochondrial surface in response to various physiological cues [42]. The morphological changes have functional implications; enhanced fusion facilitates oxidative phosphorylation and the intermitochondrial exchange of metabolites and mitochondrial DNA [43]. Fission enables more efficient mitophagy [44]. Mitophagy ensures mitochondrial quality via the clearance of damaged (e.g., impaired mitochondrial membrane potential or oxidative phosphorylation and mitochondrial DNA mutations [45]) mitochondria by autophagy and lysosomal degradation.
2.5. Mitochondrial Control of Cell Death and Survival
Mitochondria are integrally involved in the control of cellular survival. One important mechanism mediating this control is the mitochondrial membrane potential that regulates mitochondrial import and export and, upon deterioration, can trigger mitochondrial cell death signalling [53] and induce mitophagy, apoptosis, or necrosis. While the clearance of damaged or senescent cells is important to maintain a healthy cellular environment and to limit mitochondrial damage signalling, too much cell death is obviously detrimental. Neurons are in general vulnerable to imbalances in cell death signalling based on their postmitotic nature that renders them difficult to replace; this vulnerability increases with age, and certain neuronal populations are more vulnerable than others [17], which is likely an important factor in the pathogenesis of neurodegenerative diseases.
3. Improving Mitochondrial Functions by Exercise in Skeletal Muscle
The prominent health effects of regular exercise are well established and include reduced all-cause morbidity and mortality [9]. With regard to mitochondria, exercise benefits are best understood in skeletal muscle. While muscle mitochondria regulate skeletal muscle mass and function [63], they are in turn regulated by exercise. Exercise induces mitochondrial plasticity [27,64,65] and improves mitochondrial biogenesis and respiration. It also enhances antioxidant capacities and the affinity of mitochondria for oxygen [27,66,67,68,69,70], improving fatty acid oxidation, aerobic performance, health [71,72,73,74], and healthy aging [75]. These mechanisms crucially depend on the mitochondrial integrity and quality control, as well as on the mitochondria’s capacity to adequately change their morphology, increase their numbers, and enhance their mobility and distribution throughout cells.
Hormetic adaptations are thought to be important mediators of exercise-induced mitochondrial benefits [76]. “Hormesis” is a biphasic response to a stimulus (here exercise), which results in protective adaptations at low levels but that can be detrimental at high doses. It is termed “mitohormesis” if mitochondrial adaptations are concerned. Hormesis following exercise has been suggested to promote healthy brain aging [77].
Muscle mitochondrial ATP production has been shown to decrease by around 8% per 10 years of higher age in a population of 146 healthy men and women of ages between 18 and 89 years [80]. This deterioration of mitochondrial function was attributed mainly to physical inactivity [81], suggesting that regular exercise may at least partially prevent the aging-related decline of mitochondrial function. While acute exercise induces the formation of ROS, with oxidative damage occurring more frequently during high intensity bouts [82], regular moderate exercise reduces oxidative stress by bolstering the antioxidant defences of muscle tissue [30,83,84,85]. Accordingly, aging-related deficiencies in antioxidant capacities can be mitigated by life-long regular exercise [83].
4. Exercise Effects on the Brain: A Focus on Mitochondria and Oxidative Stress
The brain is well known to be responsive to regular exercise. Pronounced structural changes have recently been confirmed by the demonstration of differential brain volumes depending on exercise levels [93]. The associated aerobic fitness is further correlated with reduced brain tissue loss [94,95] and with cognitive benefits [96] during the aging process. Accordingly, exercise has emerged as an important protective factor for neurodegenerative diseases, such as AD [5] and PD [6,97].
5. Strategies to Boost Brain Mitochondria by Exercise
Although many open questions remain on the mechanistic details of the exercise-induced benefits on brain mitochondria, the potential of exercise to prevent neurodegeneration is immense. Regular exercise improves brain function, including the cognitive function [107] and hormonal activity [106] of the brain. A direct outcome of exercise—improved cardiorespiratory fitness—has been shown to protect from neurodegeneration risk factors by enhancing, for example, resilience against disruptions of CBF [237]. High cardiorespiratory fitness is also associated with reduced cognitive dysfunction in older individuals [238]. A challenge for the clinical application of exercise as a medicine is the selection of the appropriate exercise regimen, depending both on the capacities of the patients or at-risk populations and the aim. Training plans to this end should be designed with the assistance of sports science specialists [239] in order to both maximize health-promoting effects (selection of adequate exercise parameters) and exclude any risks arising, e.g., from pre-existing morbidities or exercise loads that are too high for an individual.
The WHO guidelines “Global Recommendations on Physical Activity for Health” and the recommendations from the American College of Sport Medicine (ACSM) both propose at least 150 min per week of moderate intensity (endurance) or 75 min of vigorous physical exercise (e.g., intense interval exercise) or an equivalent combination for health benefits in older adults [240,241]. Strength and balance exercise on three or more days per week is recommended for people to maintain force and to prevent falls.
Taken together, performing different types of exercises exerts specific effects on skeletal muscle mitochondria and affects performance differentially. Although this is an important characteristic to harness physical exercise as a treatment for neurological diseases, the effects of specific exercise parameters on brain and brain mitochondria are still poorly understood. Increasing the complexity of the field, the contributions of mitochondria in different brain cell types (neurons, glial cells, endothelial cells, etc.) to the beneficial effects of different exercise modalities remain to be better explored.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22126479
