Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Forestry
Due to long-term burial in the ground or water, the plant cell walls of some wooden cultural relics were degraded by microorganisms, the structure of cell wall was loose and filled with water. The moisture content of these wooden cultural relics is much higher than that of normal wood.
- Nanhai No. 1 shipwreck
- waterlogged archaeological wood (WAW)
- wood properties
1. Introduction
The Nanhai No. 1 shipwreck was a wooden merchant ship of the Southern Song Dynasty (1127 to 1279 AD), which sank in the South China Sea in Guangdong province, China. After the Nanhai No. 1 shipwreck was salvaged as a whole out of the sea, it was placed in the Maritime Silk Road Museum [1]. Many precious cultural relics had been unearthed from the Nanhai No. 1 shipwreck. Among all the cultural relics from the Nanhai No. 1 shipwreck, the wooden hull cultural relic was the most precious and the most difficult to protect, not only because the Nanhai No. 1 shipwreck is huge in volume, but also because the corrosion degree of the hull was uneven. The excavation of the wooden hull cultural relics ran through the excavation of the Nanhai No. 1 shipwreck since 2013. Maritime wooden cultural relics had been immersed in seawater for a long time, and their preservation environment was closed, at a constant temperature, constant pressure, high salt, and oxygen deficient environment. When they were excavated, the preservation environment changed into an open environment where the temperature, humidity and air circulation were not easy to control. Moreover, the control of oxidation, corrosion, microorganism biodegradation and other diseases would become extremely difficult. In order to maintain the stability of wooden cultural relics and control or delay the breeding and development of diseases, protection measures such as cleaning, moisturizing, desalination, reinforcement and anticorrosion and so on are usually taken. A series of scientific on-site protection measures laid the foundation for the transition from on-site protection of cultural relics to laboratory protection and restoration. During the excavation of the Nanhai No. 1 shipwreck, a large number of scattered individual pieces of wood were unearthed. After excavation, this hull wood was usually immersed in deionized water containing the metal chelating agent EDTA-2Na [2] and the antimicrobial agent isothiazolinone [3] for moisture stabilization, preliminary desalination, and microbial inhibition. At the same time, it is necessary to regularly monitor the water temperature, pH, concentration of main ions, conductivity, and microbial composition of the desalination buffer, and replace the desalination buffer regularly. The above treatment lays a solid foundation for the overall desalination, reinforcement, and protection of the hull in the later stage.
Wooden cultural relics are a carrier of ancient human civilization and represent a valuable material for the study of ancient history, art, science and technology, economy, and so on. Wooden cultural relics exist in many cultural sites, usually in the form of houses, tombs, hulls, decorations, and so on [4]. Wooden cultural relic can be divided into dry type and waterlogged type. In the original environment, the cellulose in the cell wall of waterlogged wooden cultural relics has been partially or completely degraded by bacteria or fungi. After the loss of degradation products, the structure of the cell wall becomes loose and even produces a large number of holes, and the original place of the cell wall is filled with water. This makes the moisture content of waterlogged archaeological wood (WAW) much higher than ordinary wood [5][6]. Generally, the moisture content of ordinary fresh wood is about 20%, while that of WAW can reach more than 500% [7][8]. The texture of WAW is fragile, so it is difficult to maintain a good condition after excavation. In general, WAW will be preserved in water after being excavated, and the temperature and humidity of the preservation environment will be as stable as possible. One is to maintain the moisture in the wood, so as to maintain the waterlogged state of the wooden cultural relic; the other is to cut off the air and avoid the further reproduction of aerobic microorganisms. The maritime wooden cultural relic is a kind of classic WAW. Due to long-term immersion in seawater, the intracellular electrolytes of maritime WAW have reached a full balance with seawater, and the wood contains a lot of salt. After the maritime WAW is excavated, many physical and chemical reactions will occur due to the change of environmental temperature and humidity, leading to the corrosion of wood. A high concentration of salts can degrade the fibers in WAW. When the environmental temperature and humidity change, some salts may crystallize and dissolve repeatedly, which will lead to fiber degradation and fracture. In addition, there are insoluble salts such as sulfur iron compounds in maritime WAW that easily oxidize into sulfuric acid during long-term exposure, leading to wood acidification and corrosion [2][9]. For example, in the protection of the Swedish warship Vasa, the problem of the acidity of wood and iron compounds in wood was highly apparent [10][11][12]. Research on the impact of biological pathways of iron and sulfur oxidization on the protection of the Mary Rose was also being studied [13]. At the same time, due to the large volume of maritime WAW, the desalination process is very slow. Therefore, it is necessary to carry out a long-term desalination treatment for maritime WAW to reduce its salt content.
During the preservation of maritime WAW, a variety of disease problems may occur. Therefore, it is necessary to regularly monitor the properties of wood, the nature of the desalination buffer, and microbial biodegradation, and replace the desalination buffer regularly. Once maritime WAW is excavated, it will come into contact with the surrounding environment, which may cause biodegradation problems. Biodegradation is mainly caused by microbial activities, mainly including bacteria and fungi, whose rapid growth and secretion of secondary metabolites may lead to damage to cultural relics [14]. Wood is an excellent organic substrate for the growth of microorganisms [15]. There are many bacteria and fungi that can produce cellulolytic enzymes and ligninolytic enzymes [16][17][18]. Some researchers analyzed the bacterial community in 108 samples of WAW from different ages and identified a variety of bacteria [19]. The main disease microorganisms of the “Xiaobaijiao No. 1” shipwreck were erosion bacteria (EB) and tunneling bacteria (TB) [15]. EB and soft rot fungi were found to be active in WAW [20].
2. Analysis of Chemical Components in the Wood
The contents of lignin, holocellulose and ash are shown in Table 1. According to literature reports, in fresh Pinus wood, the lignin content is generally about 25%, the holocellulose content is generally about 75%, and the ash content is generally less than 1% [21][22]. Through comparison, it can be found that the holocellulose content was much lower than that of fresh wood, while the ash content was higher than that of fresh wood. Since most of the cellulose had been degraded and lignin is difficult to degrade, the percentage of lignin in the wood has increased to 63%. Therefore, the wood degradation degree of WAW from the Nanhai No. 1 shipwreck was high, and the ash content of the WAW was high. In addition, the changes of lignin and holocellulose contents in April and July 2019 were not obvious, indicating that the degradation of the WAW became slow during the desalination. The ash content in July 2019 was significantly lower than that in April 2019, indicating that some inorganic salt ions were slowly removed after immersion in desalination buffer.
Table 1. Lignin, holocellulose, and ash content of WAW in NH.W2 in April and July 2019.
| Lignin Content (%) | Holocellulose Content (%) | Ash Content (%) | |
|---|---|---|---|
| 20 April 2019 | 61.32 | 4.72 | 13.02 |
| 12 July 2019 | 64.96 | 4.43 | 3.86 |
| Mean Value | 63.14 | 4.575 | 8.44 |
3. Analysis of Iron Element Content in the Buffer
The iron contents of the desalination buffer are shown in Table 2. We found that the iron content in the desalination buffer increased significantly after one month of the desalination treatment. The results showed that some insoluble salts mainly composed of sulfur iron compounds in WAW could be removed slowly after soaking in desalination treatment.
Table 2. Iron element content of the desalination buffer in NH.W2 in August and September 2019.
| Iron Element Content (g/L) | |
|---|---|
| 1 August 2019 | 127.23 |
| 10 September 2019 | 611.47 |
4. Analysis of Anion and Cation Content in the Buffer
The anion and cation content of the desalination buffer are shown in Table 3. In the original desalination buffer, Cl− and SO42− did not exist. The Na+ in the original desalination buffer mainly existed in EDTA-2Na, and the content of original Na+ was 460 mg/L. We found that after immersion in the desalination buffer, the contents of Na+, Cl− and SO42− in the desalination buffer were significantly higher than those in the original desalination buffer. Moreover, the contents of Na+, Cl− and SO42− tended to be stable after a period of desalination treatment. The results showed that the soluble salt in WAW was gradually removed after immersion in the desalination buffer.
Table 3. Anion and cation content of the desalination buffer in NH.W2 in May, June and October 2019.
| Na+ Content (mg/L) | Cl− Content (mg/L) | SO42− Content (mg/L) | |
|---|---|---|---|
| 29 May 2019 | 786.30 | 386.12 | 162.23 |
| 28 June 2019 | 745.33 | 512.67 | 219.22 |
| 22 October 2019 | 786.30 | 396.81 | 170.95 |
5. Microbial Diversity Analysis by High-Throughput Sequencing
We detected the microbial composition of the desalination buffer in NH.W2 in November 2019. Figure 1a,b show the composition and proportion of bacteria in NH.W2 desalination buffer. Figure 1a represents the distribution of the bacteria at the phylum level. The results show that at the phylum level, Proteobacteria accounts for the largest proportion, accounting for 71.95%. This is followed by Firmicutes and Actinobacteria, accounting for 13.90% and 10.04%, respectively. Figure 1b and Table 4 represent the distribution of the bacteria at the genus level. We found that at the genus level, the most abundant bacteria is Pseudomonas, accounting for 34.06%. Followed by Methylobacillus and Pandoraea, accounting for 11.33% and 7.25%, respectively. In addition, there are unidentified Mitochondria, Microbacterium, Agromyces, Leifsonia, and Escherichia-Shigella. Figure 1c,d show the composition and proportion of fungi in NH.W2 desalination buffer. Figure 1c represents the distribution of the fungi at the phylum level. The results show that at the phylum level, Basidiomycota accounts for the largest proportion, accounting for 99.63%, followed by Ascomycota, accounting for 0.25%. Figure 1d and Table 4 represent the distribution of the fungi at the genus level. We found that at the genus level, the most abundant fungi is Cutaneotrichosporon, accounting for 99.59%, followed by Fusarium and Cryptococcus, accounting for 0.18% and 0.03%, respectively.
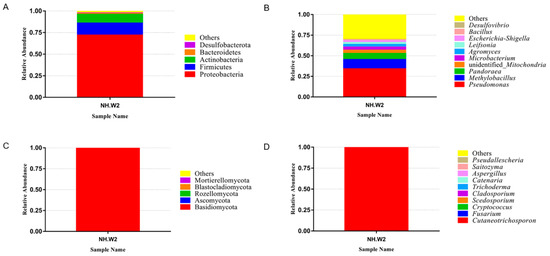
Figure 1. The relative abundance of microbial communities in monitoring tank 8 (NH. W2). The relative abundance is shown as a percentage. Phylum and genera are colored according to the legend on the right. (A) Relative abundance of bacteria at phylum level. (B) Relative abundance of bacteria at genera level. (C) Relative abundance of fungi at phylum level. (D) Relative abundance of fungi at genera level.
Table 4. Relative abundance of dominant bacteria and dominant fungi of the desalination buffer in the NH.W2 at the genus level.
| Dominant Bacteria Genus (%) | Dominant Fungi Genus (%) | ||
|---|---|---|---|
| Pseudomonas | 34.06 | Cutaneotrichosporon | 99.59 |
| Methylobacillus | 11.33 | Fusarium | 0.18 |
| Pandoraea | 7.25 | Cryptococcus | 0.03 |
| unidentified_Mitochondria | 3.99 | Scedosporium | 0.01 |
| Microbacterium | 3.75 | Cladosporium | 0.01 |
| Agromyces | 3.12 | Trichoderma | 0.01 |
| Leifsonia | 2.53 | Catenaria | 0.01 |
| Escherichia-Shigella | 2.09 | Aspergillus | 0.01 |
| Bacillus | 1.18 | Saitozyma | 0.01 |
| Desulfovibrio | 0.42 | Pseudallescheria | 0.00 |
| Others | 30.28 | Others | 0.16 |
This entry is adapted from the peer-reviewed paper 10.3390/f12050587
References
- Wei, J. 2007 whole salvage of “Nanhai No. 1”. China Cult. Herit. 2007, 4, 21–30.
- Zhang, Z.; Li, N.; Tian, X.; Liu, J.; Shen, D. Research on the removal of the iron sulfides in the Qing Dynasty marine shipwreck, Ningbo Xiaobaijiao No. 1. Sci. Conserv. Archaeol. 2014, 26, 30–38.
- Ji, H.; Zhang, L.; Liu, J.; Wang, F. Progress on Analytical Method for Isothiazolinone Derivatives Used as Industrial Biocides. Chem. Reag. 2016, 38, 523–527.
- Zheng, L.; He, Y.; Wang, Y. Study of the Restoration of Rotten Handed-down Wooden Cultural Relics. Huaxia Archaeol. 2012, 003, 136–140.
- Bjordal, C.G.; Nilsson, T.; Daniel, G. Microbial decay of waterlogged archaeological wood found in Sweden-Applicable to archaeology and conservation. Int. Biodeterior. Biodegrad. 1999, 43, 63–73.
- Capretti, C.; Macchioni, N.; Pizzo, B.; Galotta, G.; Giachi, G.; Giampaola, D. The characterization of waterlogged archaeological wood: The three roman ships found in Naples (Italy). Archaeometry 2008, 50, 855–876.
- Chen, H. Research on the correlation between water content and basic density of waterlogged wooden cultural relics. Identif. Apprec. Cult. Relics 2017, 11, 86–88.
- Li, G.; Zeng, L.; Chen, C. Study on the equilibrium water content of three kinds of hull wood of Song Dynasty ships in Quanzhou Bay and 14 kinds of modern wood in Quanzhou area. J. Fujian Coll. For. 1984, 1, 49–59.
- Hocker, E. From the Micro- to the Macro-: Managing the Conservation of the Warship, Vasa. Macromol. Symp. 2010, 238, 16–21.
- Chelazzi, D.; Giorgi, R.; Baglioni, P. Nanotechnology for Vasa Wood De-Acidification. Macromol. Symp. 2010, 238, 30–36.
- Giorgi, R.; Chelazzi, D.; Baglioni, P. Conservation of acid waterlogged shipwrecks: Nanotechnologies for de-acidification. Appl. Phys. A 2006, 83, 567–571.
- Almkvist, G.; Persson, I. Extraction of iron compounds from wood from the Vasa. Holzforschung 2006, 66, 1125–1684.
- Joanne, P.; Smith, A.D.; Schofield, E.J.; Chadwick, A.V.; Jones, M.A.; Watts, J.E.M. The Effects of Mary Rose Conservation Treatment on Iron Oxidation Processes and Microbial Communities Contributing to Acid Production in Marine Archaeological Timbers. PLoS ONE 2014, 9, 1–8.
- Sterflinger, K.; Guadalupe, P. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97.
- Gao, M.; Zhang, Q.; Jin, T.; Luo, P.; Li, Q.; Xu, R. Observation and damage assessment of microbial diseases in some wooden cultural relics from the ancient marine shipwreck, Ningbo Xiaobaijiao No. 1. Sci. Conserv. Archaeol. 2017, 29, 102–111.
- Lee, R.L.; Paul, J.W.; Willem, H.v.Z.; Isak, S. Pretorius. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577.
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194.
- OSMAN, M.E.-S.; EL-SHAPHY, A.A.E.-N.; MELIGY, D.A.; AYID, M.M. Survey for fungal decaying archaeological wood and their enzymatic activity. Int. J. Conserv. Sci. 2014, 5, 295–308.
- Eleanor, T.L.; Julian, I.M.; Hotchkiss, S.; Eaton, R.A. Bacterial diversity associated with archaeological waterlogged wood: Ribosomal RNA clone libraries and denaturing gradient gel electrophoresis (DGGE). Int. Biodeterior. Biodegrad. 2008, 61, 106–116.
- Björdal, C.G. Microbial degradation of waterlogged archaeological wood. J. Cult. Herit. 2012, 13S, S118–S122.
- Kim, B. Chemical Characteristics of Waterlogged Archaeological Wood. Holzforschung 1990, 44, 169–172.
- Passialis, C.N. Physico-Chemical Characteristics of Waterlogged Archaeological Wood. Holzforschung 1997, 51, 111–113.
This entry is offline, you can click here to edit this entry!
