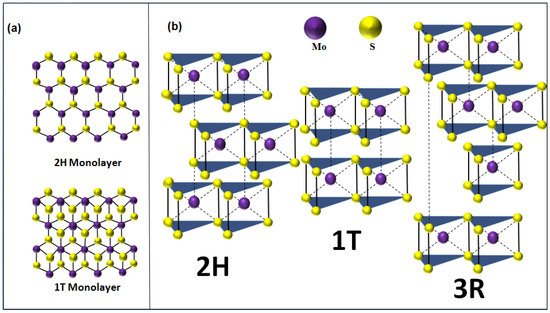
2. Fabrication Techniques of 2D-MoS2
Tremendous efforts have been devoted to the synthesis of 2D-MoS2 with controllable large-area growth and uniform atomic layers using both top-down and bottom-up approaches. The most commonly used processing routes are detailed in the following sub-sections along with their advantages and limitations.
2.1. Mechanical and Chemical Exfoliations
Mechanical exfoliation, also known as micromechanical cleavage, is a straightforward technique that takes advantage of the weak bonding between layers, for the production of high-quality mono- to few-layer MoS
2 [
58,
59,
60]. It consists of exfoliating thin films of 2D-MoS
2 from a bulk MoS
2 crystal by using a low surface tension tape to break the weak interlayer bonds in a similar way as for grapheme [
61]. Additional exfoliation of the extracted films may be needed to obtain few- to monolayer MoS
2. Tapes could be attached to glass slides to achieve planar exfoliation and slow peeling. The obtained monolayers are usually transferred to an appropriate substrate for further analysis and testing.
The advantage of the mechanical exfoliation process lies in its simplicity that requires the sole use of a confocal microscope to localize the 2D-MoS
2 layers deposited on the substrate. Conveniently, this technique can produce high crystalline quality mono- to few layers with a lateral size up to few tens of micrometers, making them highly suitable for sensing applications. However, this approach suffers from a lack of a consistent control in producing the 2D monolayers as it is heavily user-dependent and does not permit the control of the size and/or thickness uniformity of the exfoliated 2D-MoS
2 layers [
62]. Therefore, the mechanical exfoliation technique is not necessarily suitable for the production of 2D-MoS
2 layers intended for large-area and high-throughput applications.
Chemical exfoliation, on the other hand, appears as a promising approach to produce large quantities of mono- and few-layer MoS
2 nanosheets [
60,
63,
64,
65]. Eda et al. [
54] reported a high yield of monolayer crystal synthesis using chemical exfoliation of bulk MoS
2 via Li intercalation. However, this approach may induce an alteration in the quality of the produced 2D-MoS
2. For instance, the chemically exfoliated MoS
2 layers can lose their semiconducting properties because of the structural changes resulting from the Li intercalation process. However, this fabrication route stands by its ease of processing, low production costs, and suitability for catalysis and/or sensing applications [
66].
2.2. Chemical Vapor Deposition
Chemical vapor deposition (CVD) is one of the most popular routes for large-scale, high-quality, and low-cost 2D-MoS
2 material production [
49,
67,
68,
69]. CVD is a bottom-up fabrication method at the equilibrium state, which enables the processing of layered 2D-MoS
2 with controlled morphology and good crystallinity while minimizing structural defects. The control of the CVD process is ensured by tuning the deposition parameters such as temperature, pressure, gas flow rate, precursor’s quantities, and substrate types. The 2D-MoS
2 synthesis via the CVD technique can be achieved by means of thermal vapor sulfurization (TVS), thermal vapor deposition (TVD), and thermal decomposition (TD). Deokar et al. [
43] used TVS for high quality and vertically-aligned luminescent MoS
2 nanosheets. A similar process could be used to grow 2D-MoS
2 layers [
36,
70] by employing two sources, such as molybdenum thin film (below 20 nm) or molybdenum oxide (MoO
3) powder deposited on a SiO
2/Si substrate as a first precursor and the sulfur powder or gaseous sulfur source (H
2S, etc.) as the second precursor [
49,
67,
68,
69,
71,
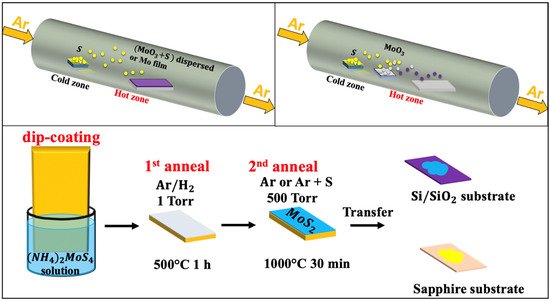
72]. A typical CVD sulfurization process (a) is usually performed in a tubular furnace reactor, where a continuous argon flow (typical flow rate 100 sccm) is used as a carrier gas to stream the evaporated sulfur into the Mo source materials.
Figure 2. Schematic of the chemical vapor deposition techniques: (
a) thermal vapor sulfurization process using a quartz tube; (
b) thermal vapor deposition process using a quartz tube; and (
c) thermal decomposition of (NH
4)
2MoS
4 (reproduced and adapted from Ref. [
34]).
One of the critical aspects to be controlled in such a CVD tubular reactor is the temperature gradient between the S powder and the substrate. In fact, while the S powder is at 150–200 °C, the substrate’s temperature—with or without Mo thin film—should be maintained in the 700–900 °C range to obtain the 2D-MoS2 phase. This technique offers sufficient latitude to fairly control the thickness and the homogeneity of the grown 2D-MoS2. The typical average lateral crystal size obtained by CVD is usually in the 10–30 nm range. shows few examples of CVD-TVS grown MoS2 nanostructures along with their associated processing conditions.
Table 2. Examples of CVD-TVS grown MoS2 nanostructures.
| Substrate |
Precursors |
Growth Conditions |
Morphology |
Ref |
| Si |
MoO3 and S powders dispersed on substrate |
MoO3 and S powders dispersed on substrate at 850 °C; S powder at 400 °C; Ar-0.725 L/min; time reaction = 30 min |
MoS2 nanosheets |
[43] |
| Si [001] |
S powder and Mo film deposited on substrate |
Mo deposited on Silicon at 850 °C, S at 400 °C; Ar-0.725 L/min; time reaction = 30 min |
MoS2 nanosheets |
[44] |
| Si/SiO2 |
S powder and Mo film deposited on substrate |
Mo deposited on Silicon at 850 °C, S at 400 °C; Ar-0.725 L/min; time reaction = 30 min |
MoS2 nanosheets |
[49] |
| Diamond substrate |
S powder and Mo deposited on substrate |
Mo deposited on Silicon with S powder at 800 °C; N2; ambient pressure; time reaction = 30 min |
Horizontally and vertically MoS2 |
[73] |
| Si/SiO2 |
S powder and MoO3 deposited on substrate |
MoO3 film deposited on Silicon at 750–850 °C, 600 mg of S powder at 100 °C; Ar-0.01 L/min; time reaction = 10 min |
Mono-to few-layers of MoS2 |
[74] |
shows the typical morphologies obtained for MoS2, which seem to depend on the carrier gas and the type of the substrate used. The reaction time and the spatial position of the substrate strongly affect the number of resulting layers.
The TVD based MoS2 growth (b) involves the concomitant evaporation of both MoO3 and S powders. This approach consists of a stepwise sulfurization of MoO3 to form the MoS2 phase. It has been shown that, by increasing the S vapor flux, the sulfurization proceeds through several phase changes before reaching the final product. First, MoO3 is formed, then MoO2 followed by MoOS2, and finally MoS2. This approach is very useful to obtain 2D MoS2 layers with a lateral size of few tens of microns. The TVD growth conditions of MoS2 under various conditions and with different characteristics are summarized in .
Table 3. Examples of TVD grown MoS2 along with their relevant processing conditions (* D is the distance between the MoO3 and S powders inside the tubular furnace).
| Substrate/Setup |
MoO3 (mg) |
S (mg) |
D * (cm) |
Gas, Flow (sccm) |
T (°C), Time (min) |
Morphology |
Ref |
| Si face-down |
15 |
80 |
18 |
Ar
10 to 500 |
700, 30 |
Flake size between 5.1–47.9 µm |
[75] |
SiO2/Si
face-up |
10 |
200 |
30 |
Ar, 100 |
850, 20 |
Monolayer, bilayer and trilayer MoS2 |
[76] |
SiO2/Si
face-down |
10 |
100 |
– |
N2, 20 |
650, 20 |
MoS2 monolayer |
[77] |
SiO2/Si
face-down |
10-30 |
– |
25 |
Ar, 150 |
800, 10 |
MoS2 triangular flakes |
[78] |
SiO2/Si
face-up |
50 |
175 |
– |
N2, 300 |
750, 15 |
MoS2 monolayer with lateral size of 50 µm |
[79] |
In comparison to the results obtained by CVD-TVS summarized in , TVD exhibits high-yield fabrication of 2D-MoS
2 monolayers generally exhibiting a triangular flakes shape. Besides, one can notice the two possible configurations of the substrate of interest in TVD face-up and face-down compared to CVD-TVS [
75,
76,
77,
78,
79].
Moreover, the TD-based CVD method presents an alternative approach to produce highly crystalline MoS
2 thin layers with superior electrical properties on insulating substrates [
34]. Typically, the TD-CVD is based on the high-temperature annealing of a thermally decomposed ammonium thiomolybdate layer (NH
4)
2MoS
4 in the presence of S, as illustrated in c. It is worth noting that the excess in sulfur introduces changes in the shape, size, and morphology of fabricated MoS
2. It also leads to a p-type MoS
2 semiconductor by increasing the electrons deficiency. In contrast, the presence of sulfur vacancies in MoS
2 was reported to have a direct impact on the catalytic properties of MoS
2, suggesting a carriers’ mobility alteration [
80]
Besides, the addition of S during the high-temperature annealing drastically enhances the crystallinity of MoS
2. Relatively, centimeter-sized MoS
2 crystals could be formed on Al
2O
3 substrates compared to SiO
2 ones [
35]. The fully covered Al
2O
3 substrate with an epitaxial monolayer of MoS
2 was achieved at 930 °C. The MoS
2 crystals nucleate in a single domain to pursue by domain-to-domain stitching process occurring during annealing at 1000 °C mediated by the oxygen flow. The difference in the self-limited monolayer growth observed between the SiO
2 and Al
2O
3 substrates is related to the absorption energy barrier on MoS
2 [
37]. In particular, the growth of MoS
2 on Al
2O
3 obeys the surface-limited epitaxial growth mode, which is not the case for the SiO
2 due to lattice mismatch. Moreover, the patterning of the as-grown MoS
2 layers has been reported by means of the polydimethylsiloxane (PDMS) stamps and the reuse of the substrate after transferring the MoS
2 layers [
35]. Recently, the epitaxial growth of centimeter wafer-scale single-crystal MoS
2 monolayers on vicinal Au (111) thin films were also obtained at a processing temperature of 720 °C, by melting and re-solidifying commercial Au foils [
36]. This allows overcoming the evolution of antiparallel domains and twin boundaries, leading to the formation of polycrystalline films. It has been proposed that the step edge of Au (111) induced the unidirectional nucleation, growth, and subsequent merging of MoS
2 monolayer domains into single-crystalline films.
2.3. Atomic Layer Deposition
The atomic layer deposition (ALD) technique is known to produce high-quality thin films even at low temperatures, typically between 150 and 350 °C. Since ALD is an atom stepwise growth process, where the reactants are alternately injected into the growth area, it allows the purging of excess species and by-products after each reaction. As a result, high-quality films are obtained by sequential surface reactions. A schematic representation of the ALD synthesis of 2D-MoS
2 can be found elsewhere [
81].
Despite the challenges related to its synthesis conditions, ALD makes it possible to deposit crystalline MoS
2 thin films at a relatively low temperature (<350 °C) followed by annealing. For instance, L.K. Tan et al. [
82] reported the possibility to use ALD for the synthesis of highly crystallized MoS
2 films on sapphire substrates at 300 °C. They prepared MoS
2 films by alternating exposure of the substrate to Mo(V) chlorides (MoCl
5) and hydrogen disulfide (H
2S) vapors. Similarly, Mattinen et al. [
83] proposed the use of a Mo based precursor, namely Mo(thd)
3 (thd = 2,2,6,6 tetramethylheptane 3,5-dionato), with H
2S as a sulfur source. They have been able to achieve a self-limiting growth and a linear film thickness control (with a very low growth rate of ≈0.025 Å per cycle). While the crystallinity of these MoS
2 films was found to be particularly good (taking into account that the deposition was done at a low temperature), their surface was rather rough, consisting of flake-like grains with a size of ≈10–30 nm. One of the advantages of this process is the possibility to deposit layered MoS
2 films on various substrates. summarizes the main processing conditions used by different groups along with the achieved MoS
2 film thicknesses.
Table 4. Summary of the ALD deposition conditions and achieved MoS2 film thicknesses.
| Substrate |
Precursors |
P (Torr) |
T (°C) |
Cycles |
Thickness |
Ref |
| SiO2/Si |
Mo hexacarbonyl and dimethyldisulfide |
1.4–3.3 |
100 |
100 |
≈11 nm |
[84] |
| SiO2/n-Si |
MoCl5 and H2S |
0.75 |
350–450 |
100 |
≈9 nm |
[85] |
| Al2O3 |
Mo(NMe2)4 and H2S |
– |
60 |
100 |
≈12 nm |
[81] |
| Al2O3 2-inch wafer |
MoCl5 and H2S |
0.001 |
300 |
50 |
≈9 nm |
[82] |
| SiO2/Si |
Mo(thd)3 (thd = 2,2,6,6 tetramethylheptane 3,5-dionato) and H2S |
3.75 |
300 |
100 |
≈25 nm |
[83] |
Al2O3
c-plane |
MoCl5 and hexamethyldisilathiane |
3.75 |
350 |
250 |
≈22 nm |
[86] |
| Carbon nanotubes, Si-wafers and glass |
bis(tbutylimino)bis(dimethylamino) Mo (VI) and H2S |
300 |
100–250 |
100 |
≈11 nm |
[87] |
| Si, SiO2, Al2O3 |
MoCl5 and H2S |
3.75 |
430–480 |
1 |
1 layer |
[88] |
| Si |
MoCl5 and H2S |
– |
390–480 |
100 |
≈21.5 nm |
[89] |
| SiO2 |
Mo hexacarbonyl and H2S |
– |
175 |
100 |
≈5 nm |
[90] |
The ALD appears as a potentially interesting technique for the production of high-quality MoS2 ultrathin films at relatively low temperatures and with the ability to achieve excellent step coverage onto different substrates. However, the very low throughput of the ALD might hinder its scalability and competitiveness in comparison with other physical and/or chemical deposition methods.
2.4. Pulsed Laser Deposition
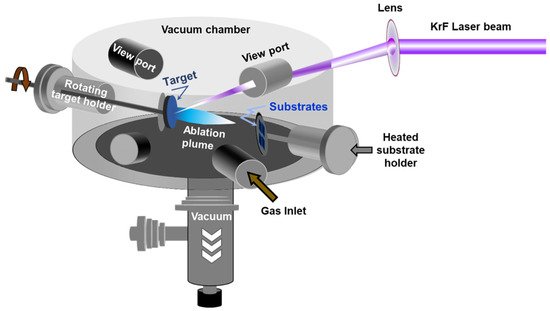
Pulsed laser deposition (PLD) has emerged as one of the most promising physical vapor deposition (PVD) techniques for the deposition of MoS2 thin films. The PLD approach consists of shining a focused high-power laser beam onto the surface of a solid target to be ablated and deposited as a film on a substrate. PLD is a non-equilibrium process that leads to the absorption of very-short (15–20 ns) and highly-energetic laser pulses by the target and to the formation of a directive plasma plume. The laser-ablated species that form the plasma plume condense onto the substrate, leading to the growth of a thin film. The PLD is well known for its large process latitude, high-flexibility, and excellent process controllability. For instance, by controlling the number of laser ablation pulses and/or the background gas pressure, nanoparticles, and/or films with thicknesses varying from few nm to few microns can be synthesized. shows a schematic representation of a PLD system.
Figure 3. Schematic of the pulsed laser deposition chamber.
Among the advantages and the unique features of the PLD method, we can cite: (i) its ability to achieve a congruent transfer to the films when a multi-element target is used [
91]; (ii) its highest instantaneous deposition rate along with the highly-energetic aspect of the ablated species (~10 times higher than in sputtering) enables the growth of metastable phases and/or crystalline phases even at room temperature; and (iii) its process latitude, which makes it easy to control almost independently each of the deposition parameters (laser intensity, number of laser ablation pulses, background gas pressure, and substrate temperature), and hence the properties of the deposited materials [
92,
93,
94]. While the early studies on the PLD of MoS
2 date back to the 1990s [
95,
96,
97,
98,
99,
100], it is only recently that important advancements have been made in PLD synthesis of 2D-MoS
2 films onto various substrates opening thereby the way to their use for different optoelectronic applications. In 2014, PLD was successfully used to grow one to several layers of MoS
2 onto different metal, semiconducting, and sapphire substrates [
101,
102]. Siegel et al. [
103] were the first to report, in 2015, the growth of MoS
2 films (from 1 to a few 10s of monolayers thick) on centimeter-sized areas. Other attempts were made to deposit ultrathin (≤3 nm) films of nearly-stoichiometric amorphous MoS
2 onto irregular surfaces such as silicon and tungsten tips and to study their field electron emission (FEE) properties [
95]. The authors stated that the addition of the MoS
2 coating is beneficial to the FEE process since lower electric fields were required to extract an electron current density of 10 μA/cm
2 (namely, 2.8 V/μm for MoS
2-coated Si and ~5.5 V/μm for MoS
2-coated W tips). More recently, PLD has been used to fabricate high-quality MoS
2 films (monolayer to few layers) and integrated them into functional ultraviolet (UV) photodetectors [
104]. The developed photodetectors were found to exhibit a very low dark current (~10 × 10
−10 A), low operating voltage (2 V), and good response time (32 ms). Their performance surpassed that previously reported for 2D-MoS
2 synthesized by other routes [
105,
106,
107,
108,
109]. Indeed, under UV irradiation, their detectivity, photoresponse (I
on/I
off ratio), and responsivity were found to be as high as 1.81 × 10
14 Jones, 1.37 × 10
5, and 3 × 10
4 A/W, respectively. summarizes most of the papers reported so far on the PLD of MoS
2 films. More specifically, it compares the main PLD growth conditions of 2D-MoS
2 films along with the obtained crystallographic phase and some of the reported optoelectronic properties.
Table 5. Summary of the PLD conditions of MoS2 films along with their thickness and some of their properties.
| Substrate |
Target |
P(Pa) |
T(°C) |
Laser Energy |
Thickness |
Properties |
Ref |
| Stainless steel |
MoS2 |
2.66 × 10−6 |
RT/200/300/450 |
5 mJ |
≈400 nm |
Granular structure stoichiometric, crystalline MoS2 |
[110] |
| Stainless steel |
MoS2 |
10−6 |
RT/300 |
100 mJ |
≈70 nm |
Stoichiometric single crystal MoS2 |
[111] |
| c-Al2O3 (0001) and Si/SiO2 |
2H-MoS2 |
9.33 × 10−4 |
600 |
500 mJ/cm2 |
≈1.4 nm |
Stoichiometric 2H phase
Flake size ≈ 10 µm |
[112] |
| GaN/c-Al2O3 (0001) |
2H-MoS2 |
8 × 10−4 |
700 |
50 mJ |
Few layers |
Mixed phase
Roughness ≈0.11 nm |
[102] |
| Titanium foil |
p-MoS2 |
1.33 × 10−2 |
RT |
– |
0.65 nm |
1T phase MoS2 |
[113] |
| SiO2 on Si [100] |
MoS2 |
1.33 × 10−2 |
800 |
200 mJ/cm2 |
≈20–60 nm |
2H phase MoS2 |
[104] |
| Gold-coated carbon cloth |
Amorphous MoS2 |
1.33 × 10−2 |
RT |
220 mJ/cm2 |
≈200 nm |
2H phase MoS2 |
[97] |
| Quartz |
MoS2 |
9 × 10−5 |
300 |
8500 mJ/cm2 |
30 layers |
Mixed phase |
[114] |
| Al2O3 (0001) |
MoS2+S
Powder |
1.33 × 10−2 |
700 |
50 mJ |
1–15 Layers of MoS2 |
p-MoS2
2H phase MoS2
Roughness of 0.27 nm |
[101] |
| Si |
MoS2 |
4 × 10−4 |
RT |
5/10/100/400 mJ/cm2 |
≈100–200 nm |
Various compositions of MoSx (x ≤ 2.2) |
[115] |
| SiO2 |
MoS2 |
3 × 10−5 |
700 |
200 mJ |
1–5 layers |
2H phase MoS2 |
[116] |
| W (100)-tip |
MoS2+poly(vinl) |
5 × 10−3 |
700 |
2000 mJ/cm2 |
≈20–60 nm |
nearly stoichiometric 2H phase MoS2 |
[95] |
| n-Si and p-Si |
MoS2+poly(vinl) |
5 × 10−3 |
700 |
500 mJ/cm2 |
≈20–60 nm |
nearly stoichiometric 2H phase MoS2 |
[95] |
| Al, Ag, Ni, Cu |
MoS2 |
2.6 × 10−5 |
500 |
50 mJ |
≈5 nm |
Epitaxial growth of 2H phase MoS2 |
[98] |
Sapphire
Quartz
SiO2
HfO2 |
MoS2 +S powder |
1.33 × 10−2 |
700 |
30 mJ |
1 monolayer—2.8 nm |
large-area growth of stoichiometric layered 2H phase MoS2 |
[117] |
| SiO2/Si |
MoS2 |
10−5 |
700 |
200 mJ |
few-layer |
2H phase MoS2 |
[118] |
| SiO2/Si |
MoS2 powder |
5 × 10−4 |
600 |
2200 mJ/cm2 |
13 nm |
Epitaxial growth of 2H phase MoS2 |
[119] |
| Si |
MoS2 |
10−4 |
RT |
100 mJ |
129–1900 nm |
Stoichiometric films |
[120] |
| c-plane sapphire |
MoS2 |
10−3 |
800 |
2000–3000 mJ/cm2 |
1–5 layers |
Epitaxial growth of 2H phase MoS2 |
[121] |
| Quartz glass |
Polycrystalline MoS2 powder |
5 × 10−4 |
300 |
8500 mJ/cm2 |
9–10 monolayers |
nearly stoichiometric 2H phase MoS2 |
[122] |
| Quartz |
MoS2 |
8.9 × 10−5 |
|
600 mJ |
≈5.8 nm |
2H phase MoS2 |
[123] |
| SiO2/Si |
MoS2@Ag |
1.33 × 10−7 |
500 |
1000–2000 mJ/cm2 |
≈1.3–12.8 nm |
2H phase MoS2 |
[124] |
| fluorophlogopite mica |
MoS2 |
10−5 |
700 |
4000 mJ/cm2 |
≈3.3 nm |
2H phase MoS2 |
[125] |
| Al2O3 (0001) |
MoS2 |
10−3 |
650 |
100 mJ |
≈400 nm |
2H phase MoS2 |
[126] |
2.5. Other Processing Routes
In addition to the main fabrication methods presented above, other PVD techniques have been used to deposit 2D-MoS
2 films. Among these methods, magnetron sputtering has been used to deposit both MoS
2 and WS
2 films onto polydimethylsiloxane (PDMS) polymer substrates [
37,
127,
128,
129,
130] with controllable defect densities. The PDMS substrate was chosen to fabricate flexible devices based on 2D-semiconducting materials. Interestingly, very smooth MoS
2 surfaces, with a roughness of less than 2 nm, were achieved by casting the polymer on a polished silicon wafer. It has also been shown that it is possible to induce subsequent crystallization of MoS
2 by exposing it to a pulsed 532 nm laser [
127].
Finally, the use of any of the above-discussed techniques to fabricate 2D-MoS2 films is mostly dictated by the availability of the equipment, expertise, and requirements of targeted application. In a general context, the physical-chemical and optoelectronic properties of the final MoS2 films will be determined to select the appropriate synthesis route. Nevertheless, the level of complexity, throughput, and fabrication costs have to be considered to choose the appropriate synthesis technique particularly when a technology has to be adopted. provides a general comparison of the preparation techniques of MoS2 described in this review by listing their main advantages and limitations.
Table 6. Comparison of the advantages and limitations of different preparation techniques of MoS2.
| Techniques |
Advantages |
Limitations |
| Mechanical exfoliation |
- -
-
High-quality and good crystallinity.
- -
-
Mono- to few-layer MoS2
- -
-
Simple process
|
- -
-
Long processing time (8–84 h)
- -
-
Tedious and no controllability
- -
-
Difficult integration with micro/optoelectronic processing
|
| Chemical exfoliation |
- -
-
Large-scale growth
- -
-
Synthesis of MoS2 monolayer
|
- -
-
Loss of semiconducting properties of MoS2 during Li intercalation.
|
| Chemical vapor deposition |
- -
-
High-quality and crystallinity
- -
-
Centimeter-scale area growth
- -
-
Good control of morphologies
|
- -
-
Caution due to the use of toxic precursors
- -
-
High synthesis temperatures requirement
- -
-
No lateral uniformity
- -
-
Mixed phases of 1T, 2H, etc.
|
| Atomic layer deposition |
- -
-
Low-temperature deposition
- -
-
Uniformity of MoS2 films
- -
-
High quality of uniformity
- -
-
Excellent step coverage
|
- -
-
Very low throughput
- -
-
Long processing time
- -
-
High cost
|
| Pulsed laser deposition |
- -
-
High-quality and faithful transfer of film stoichiometry
- -
-
Nanometer-level control of the film thickness
- -
-
Uniformity onto a large surface (up to 3” or 4” diameter wafers)
- -
-
Quasi-independent control of the growth parameters.
- -
-
Room-temperature deposition of crystallized MoS2
- -
-
Compatibility with electronic and optoelectronic device processing
|
- -
-
Relatively costly
- -
-
Presence of ablated particulates on the surface
|
| Sputtering |
- -
-
High quality and uniformity onto large surface
- -
-
Compatibility with electronic and optoelectronic device processing.
- -
-
Fair thickness control
|
- -
-
Relatively costly
- -
-
Preferential sputtering
- -
-
Less control on the stoichiometry
|
3. Characterizations of MoS2 Thin Films
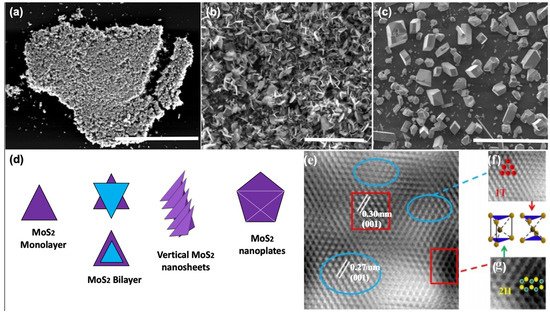
To assess the crystalline quality, microstructure, and optoelectronic properties of the synthesized 2D-MoS2, a variety of characterization techniques have been employed and reported in the literature. These include optical microscopy (OM), scanning electron microscopy (SEM), high-resolution transmission and Scanning transmission electron microscopy (HRTEM and HRSTEM), atomic force microscopy (AFM), energy-dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and photoluminescence (PL). These methods are often used to investigate the overall 2D-MoS2 surface topography and to qualify the nature of the synthesized material and the shapes of its building blocks (i.e., triangle, nanosheets, and nanoplates) (). The observations made by imaging methods are also essential to envision a possible growth mechanism of the micro/nanostructures with respect to the used processing parameters. For instance, d shows a schematic representation of the nucleation process of some morphologies of 2D-MoS2.
Figure 4. Examples of MoS
2 microstructures: (
a) planar triangle flakes scale = 40 µm; (
b) vertical nanosheets scale = 100 um; (
c) vertical nanoplates scale = 100 µm; (
d) schematic of the nucleation process of MoS
2; (
e) HRTEM image of mixed 1T-MoS
2 and 2H-MoS
2; (
f) zoom in of blue circled region of the 1T-MoS
2 structure, with the unit cell of the 1T phase; and (
g) zoom in of red circled region of the 2H-MoS
2 structure, with the unit cell of the 2H phase. (e–g adapted from Ref. [
131] Copyright 2019, Springer Nature.)
Subsequently, HRTEM investigations could be carried out to precisely characterize the MoS
2 crystalline structure and examine locally its lattice parameters and the presence of defects. In particular, the HRTEM image depicted in e is of great importance, as it was recorded in cross-region containing the two possible crystal configurations of MoS
2. As it can be seen in e–g, the identified phase mixture of 1T@2H-MoS
2 could coexist simultaneously in the same fabricated MoS
2 thin film [
131].
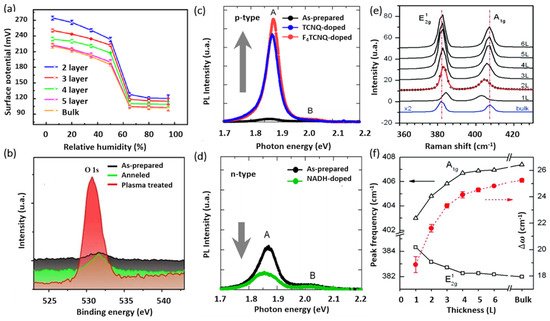
AFM and its variant methods constitute key characterization tools for the investigation of 2D crystals, mainly due to the atomically thin nature of this layered class of materials. Both vertical and lateral resolutions are fundamentally required to properly investigate the intrinsic properties of 2D materials. AFM is among the few techniques that allow the characterization of 2D-MoS2 in ambient and controlled environments at the nanometer scale. In addition to measuring the local thickness and surface topography, AFM-based electrical methods provide access to additional interesting properties such as the local variations in surface potential of 2D-MoS2. For instance, the Kelvin probe force microscopy (KPFM) method allows the characterization of the sample’s surface work function variations. The work function is an extreme surface property, which depends on the energy differences between the Fermi and vacuum levels at the surface. This renders the use of KPFM for the characterization of 2D-MoS2 fundamentally important to investigate band alignments in nanostructures and to study the dependencies of local electronic properties on the number of 2D-MoS2 layers. It also provides key insights into the environmental effects on the state of the sample surface both electronically and morphologically. The KPFM technique was used (a) to determine the surface potential variations in mono- and multilayer MoS2, under different humidity conditions.
Figure 5. (
a) Surface potential captured by KPFM vs. relative humidity RHs with respect of the number of MoS
2 layers (reproduced and adapted from Ref. [
133], Copyright 2017, IOP Publishing); (
b) XPS spectra of Mo 3d and S 2s core levels for different treatment conditions (adapted from Ref. [
132] Copyright 2014, American Chemical Society); (
c,
d) PL spectra of monolayer MoS
2 before and after being doped (reproduced from Ref. [
134] Copyright 2013, American Chemical Society); and (
e,
f) Raman spectra for various MoS
2 films with respect to the number of MoS
2 layers (reproduced from Ref. [
135] Copyright 2010, American Chemical Society).
X-ray photoelectron spectroscopy (XPS) is another relevant surface characterization technique that is widely used to achieve the elemental surface composition of MoS
2 films as well as their chemical bonding states. b shows typical high-resolution XPS spectra of the Mo
3d and S
2p core levels. The Mo
3d region exhibits two characteristic emission peaks at 232.5 (Mo 3d
3/2) and 229.4 (Mo 3d
5/2) eV. These binding energy values are consistent with electrons of Mo
4++ corresponding to MoS
2. Likewise, the S 2p
3/2 and S 2p
1/2 doublet appearing at binding energies of 162.3 and 163.5 eV is typical for S
2- in MoS
2 structure. Nan et al. [
132] used XPS to show the PL enhancement of monolayer MoS
2 through defect engineering and oxygen bonding. The chemical adsorption of oxygen created a heavy p-type doping and the conversion of the Trion into Excitons. Moreover, it caused the suppression of the non-radiative recombination of the excitons at the defect sites. Their results were verified by PL measurements at low temperature, as shown in c,d.
Unlike bulk MoS
2, the ultrathin 2D-MoS
2 (i.e., one to few layers) exhibits a strong PL intensity which increases with reducing the number of layers [
136], which has been attributed to quantum confinement effects [
53,
137]. The PL response can be tuned via several mechanisms including doping [
134], plasmonic effect, and defects engineering [
132]. For instance, Mouri et al. [
134] studied the influence of the thickness on the PL response of MoS
2 by using mono-, bi-, and trilayer MoS
2 and the PL modulation using doping. They demonstrated that p-type doping with high electron affinity seems to enhance the PL intensity, while the n-type doping tends to reduce it, as illustrated in c,d.
Moreover, Raman spectroscopy presents a very sensitive, fast, and non-destructive technique to access valuable information on the chemical structure, phase and polymorphs, crystallinity, and chemical bonding states of 2D-MoS
2 materials. It allows the monitoring of the two characteristic peaks of MoS
2, namely the in-plane and out-of-plane vibration modes E
12g and A
1g appearing for 514 nm excitation energy at the respective positions of 384.5 and 404.6 cm
−1 for 2D-MoS
2 monolayer [
135] (e). More interestingly, the difference between the peak positions of E
12g, A
1g (Δω) can be used as a robust and effective diagnostic to determine the number of MoS
2 layers (up to four layers) or to simply estimate the MoS
2 film thickness (f). Usually, Δω is less than 20 cm
−1 in the presence of a single layer of MoS
2, but it increases with increasing MoS
2 thickness to reach 25 cm
−1 for the bulk MoS
2 [
135]. In fact, a thorough study on the dependence of the characteristic Raman peak positions, width, and intensity of MoS
2 films on their thickness have been investigated [
103,
135,
138]. Furthermore, H. Li et al. [
138] reported that the frequency of the characteristic peaks is strongly dependent on the excitation energy due to the resonance effect. They showed a red shift of the E
12g mode of about 2.2 cm
−1 and blue shift of the A
1g mode of about 4.1 cm
−1. Thus, to effectively determine the exact MoS
2 number of layers using Raman spectroscopy, one has to consider the excitation energy and the thickness limit at which the Raman vibrations frequency is reaching a plateau, indicating that it is less sensitive to MoS
2 thickness variation above four layers.
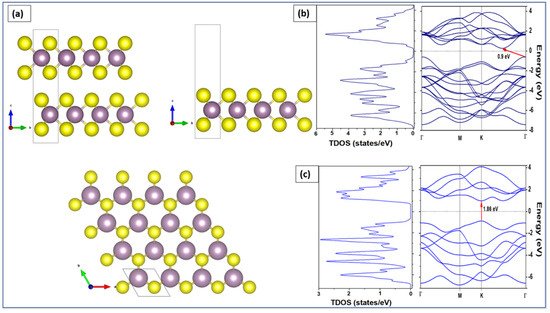
4. Band Structures and Electronic Properties
We employed density functional theory (DFT) to determine the optoelectronic properties in particular the bandgap energy of both bulk and monolayer MoS
2. Perdew–Burke–Ernzerhof (PBE) approach was applied to describe the electronic states of MoS
2 using band structure and the density of states (DOS). DFT calculations were implemented in Quantum Espresso™ code [
139,
140]. The considered 2H-MoS
2 has a hexagonal crystal form with the space group P63/mmc (No. 194). The equivalent positions for this structure employed in the calculations are Mo (1/3, 2/3, and 2/8) and S (1/3, 2/3, and 0.621). The valence electron configuration selected for Mo and S atoms are 4p
5 5s
1 and 3s
2 3p
4, respectively. The cutoff wave function and the cutoff charge densities are 70 and 700 Ryd, respectively [
140]. The cell parameters and atomic positions were fully relaxed by the process of the total energy minimization. The values of the relaxed lattice constants for bulk MoS
2 are a = 3.15 Å and c = 12.3 Å, respectively. The optimized structure was used to perform calculations for band structures and the total density of states for both MoS
2 bulk and monolayer. For bulk MoS
2 (top left panel of a), 9 × 9 ×2 k-points were used to obtain the band structure along the path Γ-K-M-Γ in the Brillouin zone. For MoS
2 monolayer (top right panel of a), 9 × 9 × 1 k-points were used. A 15 Å vacuum along the z-axis above the monolayer was added to isolate the MoS
2 and prevent any interaction between the adjacent layers [
141]. The top view of the MoS
2 monolayer is shown in the bottom panel of a, where sulfur atoms are represented in yellow and molybdenum atoms are shown in purple.
Figure 6. (a) Bulk MoS2 (top-left), monolayer MoS2 (top-right), and top view of MoS2 monolayer (bottom). Total density of states (left) and band structure (right) of the (b) bulk and (c) monolayer.
To obtain the electronic properties, the MoS
2 bulk was considered as a set of two hexagonal planes linked together by weak Van Der Waals bonds. The MoS
2 monolayer was considered as a single hexagonal plane with covalent bonds between atoms S-Mo-S [
142]. The left panel of b shows the total DOS calculation results of the bulk MoS
2 while the right panel of b shows the calculation of its band structure. The energy range is between −8 and 4 eV versus the directions of the highest symmetries in the first Brillouin zone Γ, M, K, and Γ. As observed from the band structure calculations, the MoS
2 bulk has an indirect bandgap of 0.9 eV. The minimum of the conduction band is located between K and G and the maximum of valence band at point G. This indirect bandgap obtained for the MoS
2 bulk was attributed to the presence of interlayer interactions in the bulk structure [
143]. In contrast, c shows that the monolayer MoS
2 has a direct bandgap of 1.89 eV at the K point. The DOS results are compatible with the results of the band structure. Similar conclusions have been stated in other investigations [
141,
142].